O4126
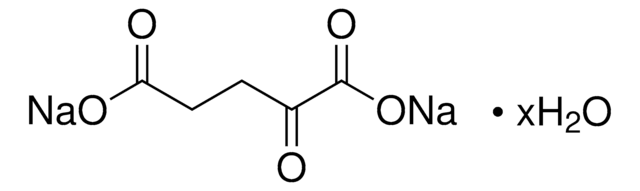
Oxaloacetic acid
≥97% (HPLC)
Synonym(s):
2-Oxosuccinic acid, Ketosuccinic acid, Oxalacetic acid, Oxobutanedioic acid
About This Item
Recommended Products
Quality Level
Assay
≥97% (HPLC)
form
powder
solubility
H2O: 100 mg/mL, clear to slightly hazy, colorless to light yellow
storage temp.
−20°C
SMILES string
OC(=O)CC(=O)C(O)=O
InChI
1S/C4H4O5/c5-2(4(8)9)1-3(6)7/h1H2,(H,6,7)(H,8,9)
InChI key
KHPXUQMNIQBQEV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Biochem/physiol Actions
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Learn about monosaccharide biosynthesis and the metabolism of monosaccharides. A unit of a carbohydrate and the simplest form of a sugar, a monosaccharide cannot be hydrolyzed into a simpler compound.
Get to know the Tricarboxylic acid (TCA) cycle to better inform your research in biochemistry, metabolomics, or related fields concerned with this metabolic pathway and its enzymes, by-products, or intermediates.
Sigma-Aldrich presents an article about how proliferatively active cells require both a source of carbon and of nitrogen for the synthesis of macromolecules. Although a large proportion of tumor cells utilize aerobic glycolysis and shunt metabolites away from mitochondrial oxidative phosphorylation, many tumor cells exhibit increased mitochondrial activity.
Information on fatty acid synthesis and metabolism in cancer cells. Learn how proliferatively active cells require fatty acids for functions such as membrane generation, protein modification, and bioenergetic requirements. These fatty acids are derived either from dietary sources or are synthesized by the cell.
Protocols
Malic dehydrogenase from bovine heart contains a histidine residue at the NAD-binding active site that is critical for activity. This protocol uses a spectrophotometric assay to evaluate malic dehydrogenase activity.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service