MSQC11
SILu™MAb Adalimumab Stable-Isotope Labeled Monoclonal Antibody
Synonym(s):
Mass spectrometry standard, Adalimumab, SIL Adalimumab, Stable isotope labelled Adalimumab
About This Item
Recommended Products
recombinant
expressed in CHO cells
Quality Level
antibody product type
primary antibodies
Assay
≥90% (SDS-PAGE)
packaging
vial of 100 μg
shipped in
wet ice
storage temp.
−20°C
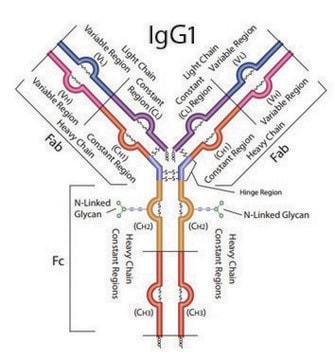
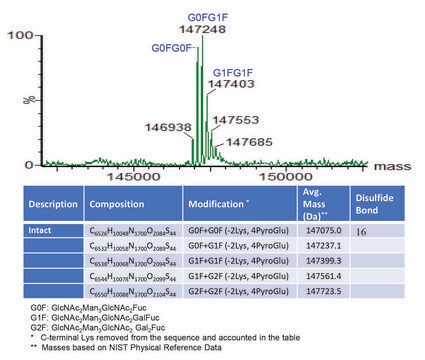
General description
SILu™ MAb Adalimumab is for R&D use only. Not for drug, household, or other uses.
Immunogen
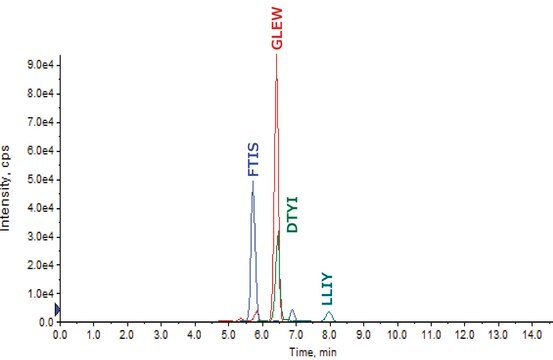
FTISRDNAKNSLYLQMNSLRAEDTAVYYCAKVSYLSTASSLDYWGQGTLVTVSSASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWNS
GALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEV
TCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSRD
ELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPG
SILu™Mab Adalimumab Light Chain:
DIQMTQSPSSLSASVGDRVTITCRASQGIRNYLAWYQQKPGKAPKLLIYAASTLQSGVPSRFSGSGSGTDFTLTISSLQPEDVATYYCQRYNRAPYTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVYACEVTHQGLSSPVTKSFNRGEC
Application
Sequence
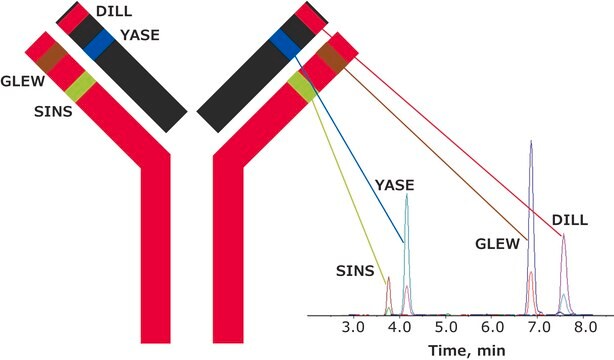
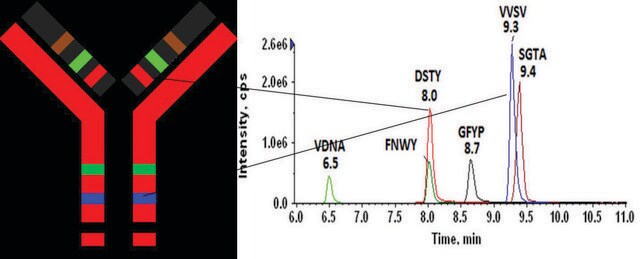
Universal Peptide Sequence Location
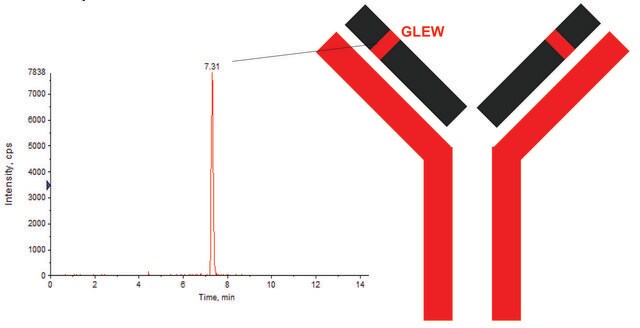
GLEWVSAITWNSGHIDYADSVEGRHeavy chain
APYTFGQGTKLight chain
FSGSGSGTDFTLTISSLQPEDVATYYCQRLight chain
Preparation Note
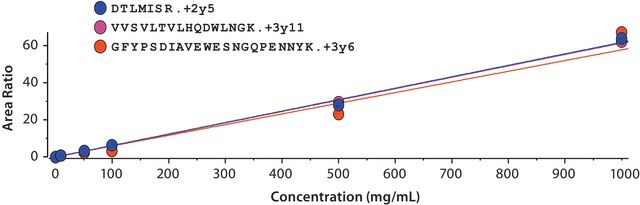
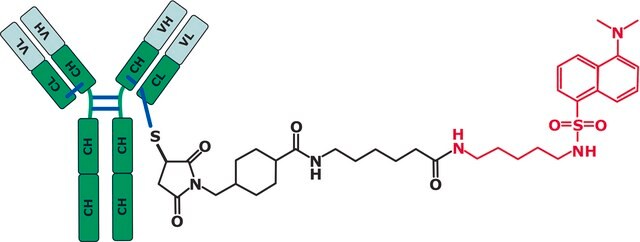
SILu™Mab Adalimumab is designed to be used as a internal standard for analysis of Adalimumab in human serum.
Reconstitution
- Briefly centrifuge the vial at ~10,000 × g to collect the product at the bottom of the vial.
- Add 500 μL of ultrapure water containing 0.1% formic acid to the vial.
- Mix the contents by gently inverting the vial a minimum of 5 times.
- Allow the vial to stand at room temperature for at least 15 minutes and repeat mixing by inversion.
Analysis Note
MRM settings provided (xls)
Legal Information
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Infliximab and Adalimumab monoclonal antibodies (mAbs) are used widely to treat rheumatoid arthritis, psoriatic arthritis, and many autoimmune diseases by binding to tumor necrosis factor-alpha (TNFα) to reduce the inflammatory response.
Development of Simple and Rapid Workflows for Quantitation of Infliximab and Adalimumab in Human Serum by LC-MS/MS
Protocols
An optimized LC-MS/MS based workflow for low artifact tryptic digestion and peptide mapping of monoclonal antibody, adalimumab (Humira) using filter assisted sample preparation (FASP).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service