D9150
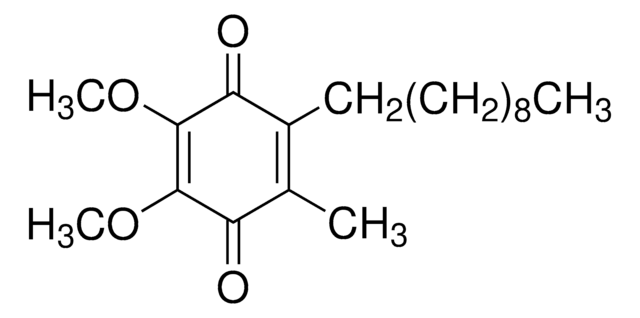
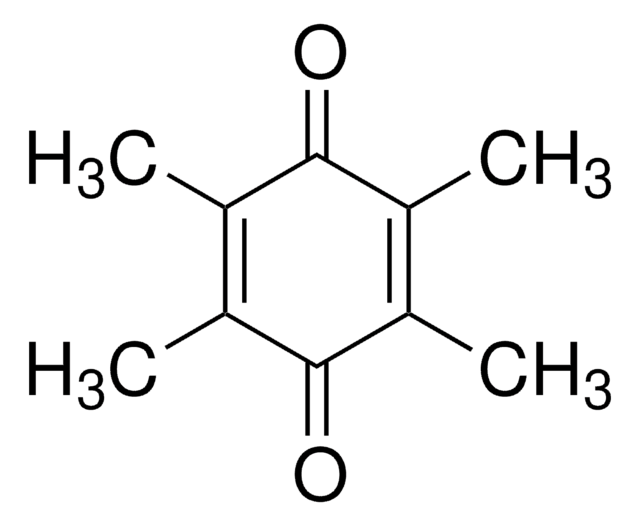
2,3-Dimethoxy-5-methyl-p-benzoquinone
apoptosis inducer
Synonym(s):
Coenzyme Q0
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C9H10O4
CAS Number:
Molecular Weight:
182.17
Beilstein:
1640422
EC Number:
MDL number:
UNSPSC Code:
12352204
PubChem Substance ID:
NACRES:
NA.51
Recommended Products
form
powder
mp
58-60 °C (lit.)
storage temp.
2-8°C
SMILES string
COC1=C(OC)C(=O)C(C)=CC1=O
InChI
1S/C9H10O4/c1-5-4-6(10)8(12-2)9(13-3)7(5)11/h4H,1-3H3
InChI key
UIXPTCZPFCVOQF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,3-Dimethoxy-5-methyl-p-benzoquinone (Coenzyme Q0 or DMM) is present in all the cells including neural cells.
Application
2,3-Dimethoxy-5-methyl-p-benzoquinone has been used:
- as a tau protein fibrillization inducer to determine the regions of tau involved in the formation of paired helical filaments (PHFs)
- as a component in buffer B for cytochrome oxidation assay with subsaturating light
- in the RPMI-1640 medium for 2,3-bis-(2-methoxy-4-nitro-5-sulphenyl)-(2H)-tetrazolium-5-carboxanilide (XTT) assay to quantify antifungal activity
Coenzyme Q0 inhibits (via radical quenching) reactions of gamma-irradiation induced homolytic cleavage of O-glycoside bonds in polysaccharides. Coenzyme Q0 induces apoptosis and modulates the cell cycle in estrogen receptor negative breast cancer cells. It is toxic to other cells such as insulin producing cells.
Biochem/physiol Actions
2,3-Dimethoxy-5-methyl-p-benzoquinone (Coenzyme Q0) interacts with tau protein and aids in the formation of filamentous structure.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
XTT assay of antifungal activity
Loures FV and Levitz SM
PLoS Pathogens, 5(15), e1543-e1543 (2015)
The Photosynthetic Bacterial Reaction Center: Structure and Dynamics, 114-114 (2013)
Augustin C Mot et al.
PloS one, 15(1), e0225530-e0225530 (2020-01-22)
Yellow laccases lack the typical blue type 1 Cu absorption band around 600 nm; however, multi-copper oxidases with laccase properties have been reported. We provide the first evidence that the yellow laccase isolated from Sclerotinia sclerotiorum is obtained from a
In vitro tau fibrillization: mapping protein regions
Santa-Maria I, et al.
Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 1762(7), 683-692 (2006)
Yung-Fu Wang et al.
Bioelectrochemistry (Amsterdam, Netherlands), 69(1), 74-81 (2006-01-25)
Bioelectrocatalytic oxidation of acetate was investigated under anaerobic conditions by using Escherichia coli K-12 (IFO 3301) cells cultured on aerobic media containing poly-peptone, glucose or acetate as the sole carbon source. It was found that all E. coli cells cultured
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service