80645
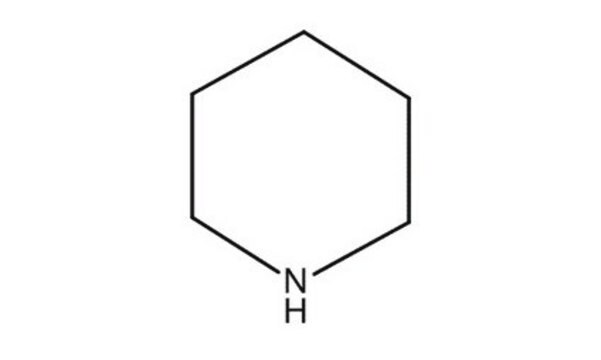
Piperidine solution
suitable for peptide synthesis, 20% in DMF
Synonym(s):
Azacyclohexane, Cyclopentimine, Hexahydropyridine, Pentamethyleneimine
About This Item
Recommended Products
form
liquid
Quality Level
concentration
20% in DMF
impurities
≤0.1% water
refractive index
n20/D 1.434
density
0.930 g/mL at 20 °C
application(s)
peptide synthesis
SMILES string
C1CCNCC1
InChI
1S/C5H11N/c1-2-4-6-5-3-1/h6H,1-5H2
InChI key
NQRYJNQNLNOLGT-UHFFFAOYSA-N
Application
- Targeted potent antimicrobial and antitumor oxygen-heterocyclic-based pyran analogues: synthesis and computational studies.: This research highlights the application of piperidine solution in the synthesis of novel pyran analogues, demonstrating its utility in enhancing antimicrobial and antitumor properties through advanced chemical synthesis and computational modeling techniques (El-Wahab et al., 2024).
- Heterocyclic Amine-Induced Feeding Deterrence and Antennal Response of Honey Bees.: Utilizing piperidine solution, this study examines the impact of heterocyclic amines on honey bees, providing valuable insights into the chemical ecology of plant-insect interactions and potential strategies for pest control in agriculture (Larson et al., 2021).
- Effects of Desilication in NaOH/Piperidine Medium and Phosphorus Modification on the Catalytic Activity of HZSM-5 Catalyst in Methanol to Propylene Conversion.: This study uses piperidine solutions to modify the surface properties of HZSM-5 catalysts, significantly enhancing their performance in methanol to propylene conversion processes, highlighting the application in industrial catalysis (Safaei et al., 2021).
- MWW-Type Titanosilicate Synthesized by Simply Treating ERB-P Zeolite with Acidic H(2)TiF(6) and Its Catalytic Performance in a Liquid Epoxidation of 1-Hexene with H(2)O(2).: This research utilizes piperidine solution in the synthesis of novel titanosilicate materials, exploring its effectiveness in the catalytic epoxidation of hexene, demonstrating the potential of piperidine in facilitating advanced material synthesis (Guo et al., 2020).
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Eye Dam. 1 - Flam. Liq. 3 - Repr. 1B - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
82.4 °F - closed cup
Flash Point(C)
28 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
KitAlysis™ Cu C-N (Buchwald-Hartwig) cross-coupling high-throughput screening kit. Detailed Set-Up User Guide and a downloadable excel file for calculations.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service