8.55079
DHP HM resin (100-200 mesh)
Novabiochem®
Synonym(s):
3,4-Dihydro-2H-pyran-2-yl-methoxymethyl polystyrene (100-200 mesh), Ellman′s dihydropyran resin
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12162002
Recommended Products
Quality Level
product line
Novabiochem®
form
beads
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
reactivity: alcohol reactive
manufacturer/tradename
Novabiochem®
application(s)
peptide synthesis
storage temp.
2-8°C
Related Categories
General description
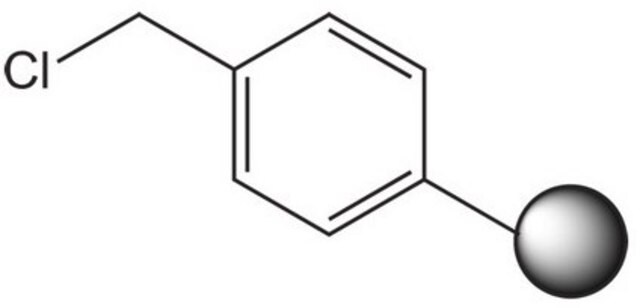
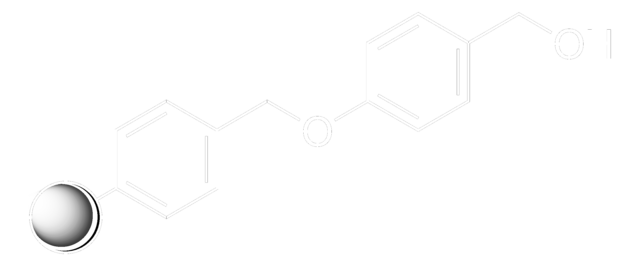
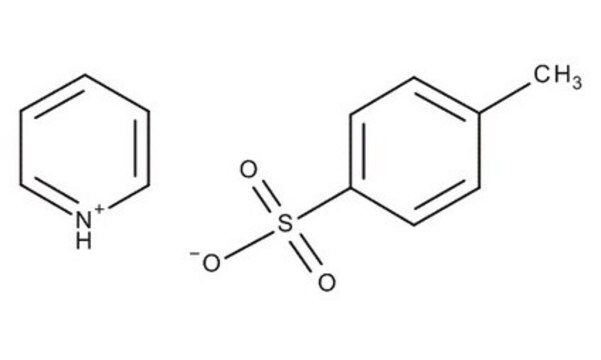
A highly versatile support for the solid phase immobilization of primary, secondary alcohols [1,2,3,4,5,6,7,8,9,10,11], phenols [12], purines [13], and indoles [14]. Derivatization is effected by treating the support in DCM with an excess of alcohol in the presence of a catalytic amount of pyridinium p-toluenesulfonate. A similar DHP-based support has also been used for the immobilization of tetrazoles [15].Release of the product alcohol from the support has been carried out by treating with 95% TFA/5% water[1] and TFA/DCM/EtOH [2,12]. However, the use of TFA can lead, in some cases, to trifluoroacetate formation. Methods which eliminate this potential side reaction include PPTS/BuOH/DCE [3], TosOH in DCM [16] and TosOH in DCE/BuOH [17].
Associated Protocols and Technical Articles
Protocols for Loading of Peptide Synthesis Resins
Literature references
[1] L. A. Thompson & J. A. Ellman (1994) Tetrahedron Lett., 35, 9333.
[2] O. B. Wallace (1997) Tetrahedron Lett., 38, 4939.
[3] G. Liu & J. A. Ellman (1995) J. Org. Chem., 60, 7712.
[4] E. K. Kick & J. A. Ellman (1995) J. Med. Chem., 38, 1427.
[5] J. S. Koh & J. A. Ellman (1996) J. Org. Chem., 61, 4494.
[6] J. Cossy, et al. (2000) Synlett, 3, 409.
[7] M. Ramaseshan, et al. (2000) J. Comb. Chem., 2, 615.
[8] M. Ramaseshan, et al. (2000) Tetrahedron Lett., 41, 4743.
[9] A. Bianco, et al. (2000) J. Org. Chem., 65, 2179.
[10] M. Steger, et al. (2001) Bioorg. Med. Chem. Lett., 11, 2537.
[11] A. Dahlgren, et al. (2003) Bioorg. Med.Chem. Lett., 11, 827.
[12] W. H. Pearson & R. B. Clark (1997) Tetrahedron Lett., 38, 7669.
[13] D. A. Nugiel, et al. (1997) J. Org. Chem., 62, 201.
[14] A. L. Smith, et al. (1998) Tetrahedron Lett., 39, 8317.
[15] S.-E. Yoo, et al. (1997) Tetrahedron Lett., 38, 1203.
[16] J. Beythien (Merck Biociences AG), personal communication.
[17] M. R. Tremblay, et al. (1999) Bioorg. Med . Chem. Lett., 9, 2827.
Associated Protocols and Technical Articles
Protocols for Loading of Peptide Synthesis Resins
Literature references
[1] L. A. Thompson & J. A. Ellman (1994) Tetrahedron Lett., 35, 9333.
[2] O. B. Wallace (1997) Tetrahedron Lett., 38, 4939.
[3] G. Liu & J. A. Ellman (1995) J. Org. Chem., 60, 7712.
[4] E. K. Kick & J. A. Ellman (1995) J. Med. Chem., 38, 1427.
[5] J. S. Koh & J. A. Ellman (1996) J. Org. Chem., 61, 4494.
[6] J. Cossy, et al. (2000) Synlett, 3, 409.
[7] M. Ramaseshan, et al. (2000) J. Comb. Chem., 2, 615.
[8] M. Ramaseshan, et al. (2000) Tetrahedron Lett., 41, 4743.
[9] A. Bianco, et al. (2000) J. Org. Chem., 65, 2179.
[10] M. Steger, et al. (2001) Bioorg. Med. Chem. Lett., 11, 2537.
[11] A. Dahlgren, et al. (2003) Bioorg. Med.Chem. Lett., 11, 827.
[12] W. H. Pearson & R. B. Clark (1997) Tetrahedron Lett., 38, 7669.
[13] D. A. Nugiel, et al. (1997) J. Org. Chem., 62, 201.
[14] A. L. Smith, et al. (1998) Tetrahedron Lett., 39, 8317.
[15] S.-E. Yoo, et al. (1997) Tetrahedron Lett., 38, 1203.
[16] J. Beythien (Merck Biociences AG), personal communication.
[17] M. R. Tremblay, et al. (1999) Bioorg. Med . Chem. Lett., 9, 2827.
Linkage
Replaces: 01-64-0192
Analysis Note
Color (visual): slight yellow to beige
Appearance of substance (visual): beads
Loading (determined from the substitution of the Fmoc-Gly-ol loaded resin): 0.70 - 1.20 mmol/g
Swelling Volume (in CH₂Cl₂): lot specific result
The polymer matrix is copoly (styrene-1% DVB), 100 - 200 mesh
Appearance of substance (visual): beads
Loading (determined from the substitution of the Fmoc-Gly-ol loaded resin): 0.70 - 1.20 mmol/g
Swelling Volume (in CH₂Cl₂): lot specific result
The polymer matrix is copoly (styrene-1% DVB), 100 - 200 mesh
Legal Information
Novabiochem is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service