8.52351
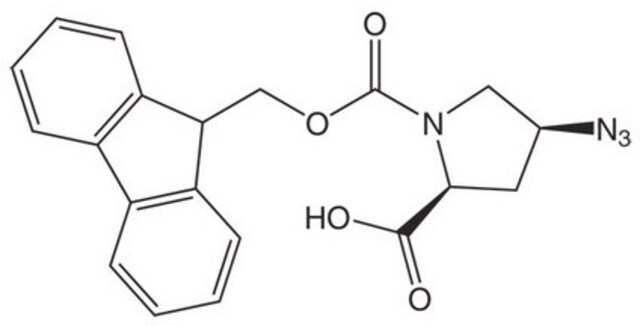
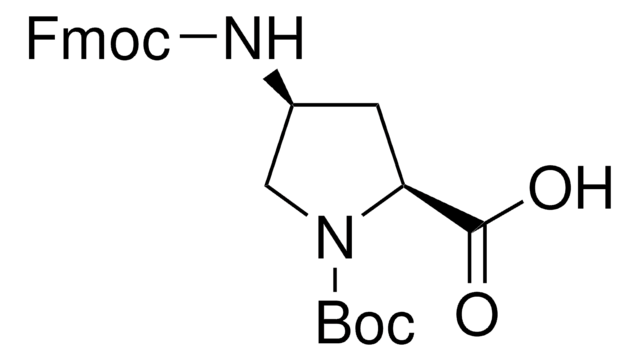
cis-Fmoc-Pro(4-N3)-OH
≥98.0% (HPLC), for peptide synthesis, Novabiochem®
Synonym(s):
cis-Fmoc-Pro(4-N3)-OH, Fmoc-(2S, 4S)-4-azidoproline
About This Item
Recommended Products
product name
cis-Fmoc-Pro(4-N3)-OH, Novabiochem®
Quality Level
product line
Novabiochem®
Assay
≥98.0% (HPLC)
form
powder
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
manufacturer/tradename
Novabiochem®
application(s)
peptide synthesis
functional group
azide
storage temp.
15-25°C
InChI
1S/C20H18N4O4/c21-23-22-12-9-18(19(25)26)24(10-12)20(27)28-11-17-15-7-3-1-5-13(15)14-6-2-4-8-16(14)17/h1-8,12,17-18H,9-11H2,(H,25,26)/t12-,18-/m0/s1
InChI key
HOPXMBBEYJTPNX-SGTLLEGYSA-N
Related Categories
General description
Associated Protocols and Technical Articles
Guide to Selection of Orthogonally-Protected Building Blocks for Fmoc SPPS
Literature references
[1] M. Meldal, et al. (1997) Tetrahedron Lett., 38, 2531.
[2] J. T. Lundquist & J. C. Pelletier (2001) Org. Lett.,3, 781.
[3] N. Nepomniaschiy, et al. (2008) Org.Lett., 10, 5243.
Analysis Note
Appearance of substance (visual): powder
Identity (IR): passes test
Assay (HPLC, area%): ≥ 98.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Proline analogues are promising candidates for tuning the biological, pharmaceutical, or physicochemical properties of naturally occuring, as well as de novo designed, linear, and, cyclic peptides.
Novabiochem® product range has one of the largest collections of orthogonally and quasi-orthogonally protected tri-functional amino acids. These derivatives are useful tools for the synthesis of cyclic and branched peptides and peptides carrying side-chain modifications.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service