W267511
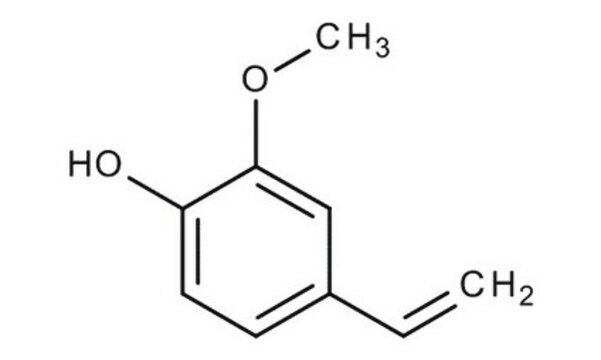
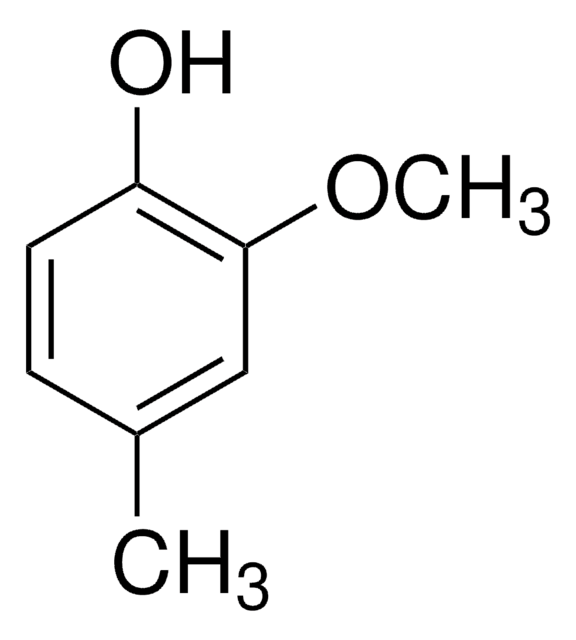
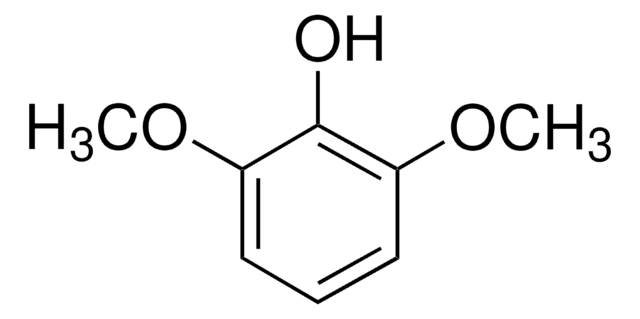
2-Methoxy-4-vinylphenol
≥98%, FG
Synonym(s):
4-Vinyl guaiacol
About This Item
Recommended Products
biological source
synthetic
Quality Level
grade
FG
Fragrance grade
Halal
Kosher
Agency
follows IFRA guidelines
meets purity specifications of JECFA
reg. compliance
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 117
FDA 21 CFR 172.515
Assay
≥98%
refractive index
n20/D 1.582 (lit.)
bp
224 °C (lit.)
density
1.11 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
fragrance allergen
no known allergens
Organoleptic
cedar; woody; peanut
SMILES string
COc1cc(C=C)ccc1O
InChI
1S/C9H10O2/c1-3-7-4-5-8(10)9(6-7)11-2/h3-6,10H,1H2,2H3
InChI key
YOMSJEATGXXYPX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Anti-Ulcerative Colitis Effects and Active Ingredients in Ethyl Acetate Extract from Decoction of Sargentodoxa cuneata.: This research explores the therapeutic potential of Sargentodoxa cuneata in treating ulcerative colitis, highlighting 2-Methoxy-4-vinylphenol as one of its active ingredients. It offers valuable data for chemists working on new treatments for inflammatory diseases (Yu et al., 2023).
- Phytochemical characterization, anti-diarrhoeal, analgesic, anti-inflammatory activities and toxicity profile of Ananas comosus (L.) Merr (pineapple) leaf in albino rats.: This comprehensive study assesses the medicinal properties of pineapple leaf, identifying 2-Methoxy-4-vinylphenol among its phytochemicals. It supports further research into its use for pain relief and anti-inflammatory purposes (Ugbogu et al., 2024).
Packaging
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service