M89617
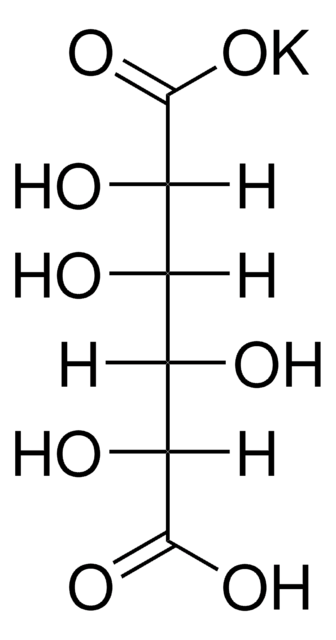
Mucic acid
97%
Synonym(s):
Galactaric acid, MTPA, Saccharolactic acid, Tetrahydroxyadipic acid, Tetrahydroxyhexanedioic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
HOOC(CHOH)4COOH
CAS Number:
Molecular Weight:
210.14
Beilstein:
1728117
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
mp
220-225 °C (dec.) (lit.)
SMILES string
O[C@@H]([C@@H](O)[C@H](O)C(O)=O)[C@@H](O)C(O)=O
InChI
1S/C6H10O8/c7-1(3(9)5(11)12)2(8)4(10)6(13)14/h1-4,7-10H,(H,11,12)(H,13,14)/t1-,2+,3+,4-
InChI key
DSLZVSRJTYRBFB-DUHBMQHGSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Mucic acid or galactaric acid can be used as a precursor for the synthesis of:
It can be also utilized in the surface modification of monodisperse water-soluble magnetic nanoparticles.
- 2,3,4,5-tetra-O-acetylgalactaric acid (AGA) which is used as a monomer unit along with adipic acid for the synthesis of copolyanhydrides.
- Muconic acid and adipic acid.
It can be also utilized in the surface modification of monodisperse water-soluble magnetic nanoparticles.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Highly Efficient Chemical Process To Convert Mucic Acid into Adipic Acid and DFT Studies of the Mechanism of the Rhenium-Catalyzed Deoxydehydration
Li X, et al.
Angewandte Chemie (International ed. in English), 53(16), 4200-4204 (2014)
Synthesis and characterization of copolyanhydrides of carbohydrate-based galactaric acid and adipic acid
Mehtio T, et al.
Carbohydrate Research, 402, 102-110 (2015)
Functionalization of monodisperse magnetic nanoparticles
Lattuada M and Hatton T A
Langmuir, 23(4), 2158-2168 (2007)
Yemin Liu et al.
Journal of the American Chemical Society, 127(9), 3004-3015 (2005-03-03)
Nucleic acid drugs have great potential to treat many devastating aliments, but their application has been hindered by the lack of efficacious and nontoxic delivery vehicles. Here, a new library of poly(glycoamidoamine)s (D1-D4, G1-G4, and M1-M4) has been synthesized by
The unusually stable crystal structure of neo-inositol.
S J Angyal et al.
Carbohydrate research, 263(1), 149-154 (1994-10-03)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service