E35400
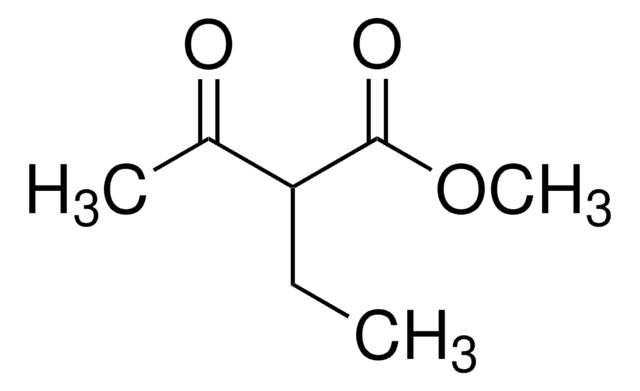
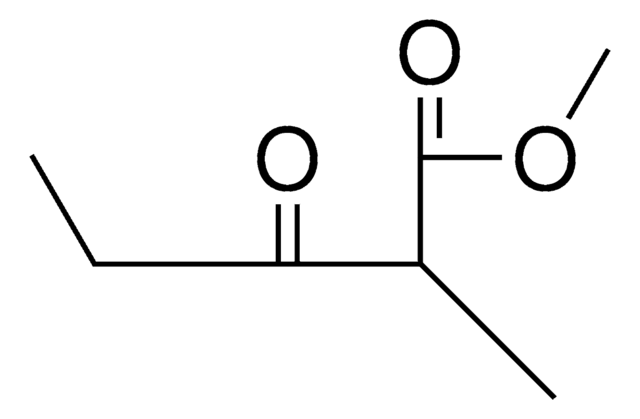
Ethyl 2-methylacetoacetate
90%
Synonym(s):
Ethyl 2-methyl-3-oxobutanoate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3COCH(CH3)CO2C2H5
CAS Number:
Molecular Weight:
144.17
Beilstein:
1071742
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
90%
form
liquid
refractive index
n20/D 1.418 (lit.)
bp
187 °C (lit.)
density
1.019 g/mL at 25 °C (lit.)
SMILES string
CCOC(=O)C(C)C(C)=O
InChI
1S/C7H12O3/c1-4-10-7(9)5(2)6(3)8/h5H,4H2,1-3H3
InChI key
FNENWZWNOPCZGK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Ethyl 2-methylacetoacetate is used as a substrate in the rhenium-catalyzed synthesis of multisubstituted aromatic compounds.
- It can be employed in the synthesis of coumarin derivatives via Pechmann condensation.
- It undergoes dehydration to yield conjugated alkynyl and allenyl esters.
- It is also used in the total synthesis of chlorotonil A, yangjinhualine A, (+)- and (−)-saudin.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
145.4 °F - closed cup
Flash Point(C)
63 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Selective One-Pot Synthesis of Allenyl and Alkynyl Esters from β-Ketoesters.
Maity P and Lepore S D
The Journal of Organic Chemistry, 74(1), 158-162 (2008)
The total synthesis of chlorotonil A.
Rahn N and Kalesse M
Angewandte Chemie (International Edition in English), 47(3), 597-599 (2008)
Rhenium-Catalyzed Synthesis of Multisubstituted Aromatic Compounds via C? C Single-Bond Cleavage.
Kuninobu Y, et al.
Organic Letters, 10(14), 3133-3135 (2008)
T Kuramoto et al.
Bioscience, biotechnology, and biochemistry, 63(3), 598-601 (1999-05-05)
Chlorella pyrenoidosa Chick reduced ethyl 2-methyl 3-oxobutanoate to the corresponding alcohols with the diastereomer (anti/syn) ratio of 53/47. The enantiomer excesses of anti-(2S, 3S)- and syn-(2S, 3R)-hydroxy esters were 89 and > 99ee% respectively. C. vulgaris and C. regularis afforded
Synthesis of coumarins catalyzed by eco-friendly W/ZrO2 solid acid catalyst.
Reddy B M, et al.
Synthetic Communications, 31(23), 3603-3607 (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service