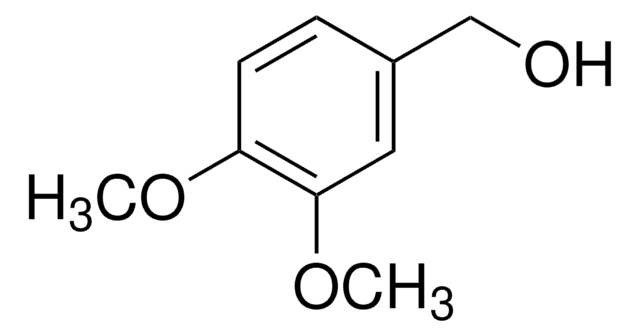
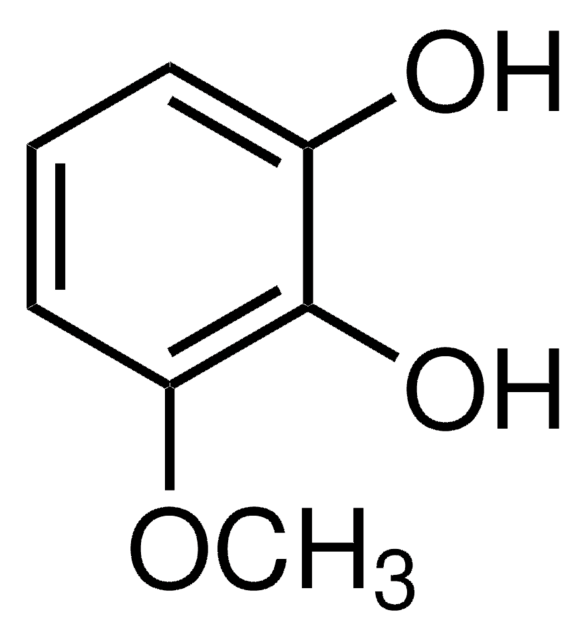
D135550
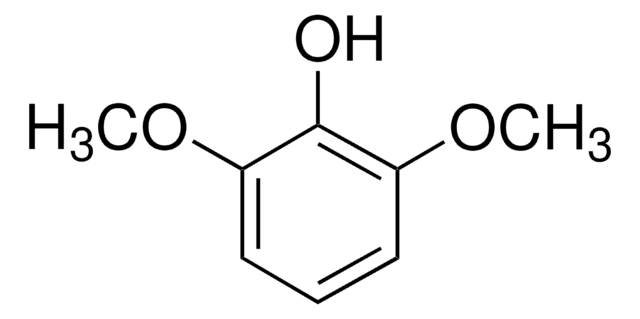
2,6-Dimethoxyphenol
99%
Synonym(s):
Pyrogallol 1,3-dimethyl ether
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3O)2C6H3OH
CAS Number:
Molecular Weight:
154.16
Beilstein:
1526871
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
bp
261 °C (lit.)
mp
50-57 °C (lit.)
SMILES string
COc1cccc(OC)c1O
InChI
1S/C8H10O3/c1-10-6-4-3-5-7(11-2)8(6)9/h3-5,9H,1-2H3
InChI key
KLIDCXVFHGNTTM-UHFFFAOYSA-N
Gene Information
human ... GABRA1(2554)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2,6-Dimethoxyphenol, also known as Syringol, is an aromatic compound that belongs to the class of methoxyphenols. It is commonly used as a component and precursor during the synthesis of dimers, vanillin, and azo dyes.
Application
2,6-Dimethoxyphenol is utilized as a building block in the synthesis of 1,3-bis(4-hydroxy-3,5-dimethoxyphenyl) adamantane via condensation reaction.
- Synthesis and Antioxidant Ability of 5-amino-1, 3, 4-oxadiazole Derivatives Containing 2, 6-dimethoxyphenol: This study reports the synthesis of new antioxidant materials incorporating 2,6-dimethoxyphenol (KF Ali, 2015).
- Catalytic cleavage of the CO bond in 2, 6-dimethoxyphenol: This research explores the non-solvent catalytic conversion of 2,6-dimethoxyphenol, a model lignin compound, highlighting a sustainable approach to biomass utilization (P Yu et al., 2020).
- Synthesis and antioxidant ability of new 5-amino-1, 2, 4-triazole derivatives containing 2, 6-dimethoxyphenol: The synthesis of new derivatives aimed at improving antioxidant properties, demonstrating the chemical versatility of 2,6-dimethoxyphenol (DF Hussain, 2016).
- Electrochemical Characterization of the Laccase-Catalyzed Oxidation of 2, 6-Dimethoxyphenol: This study provides an electrochemical insight into the enzymatic oxidation processes of 2,6-dimethoxyphenol, relevant for biotechnological applications (GJ Mattos et al., 2022).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
284.0 °F - closed cup
Flash Point(C)
140 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A Miele et al.
Journal of applied microbiology, 108(3), 998-1006 (2009-09-09)
To select better performing laccase variants among the 2300 randomly mutated variants of Pleurotus ostreatus POXA1b laccase to develop improved laccase-based biocatalysts. Screening of collections of 2300 randomly mutated variants of POXA1b was performed by assaying activity towards the phenolic
Yu Huan Liu et al.
Applied microbiology and biotechnology, 91(3), 667-675 (2011-04-28)
To obtain better performing laccases for textile dyes decolorization, random mutagenesis of Lac591, a metagenome-derived alkaline laccase, was carried out. After three rounds of error-prone PCR and high-throughput screening by assaying enzymatic activity toward the phenolic substrate 2,6-dimethoxyphenol (2,6-DMP), a
Eduardo Coelho et al.
Molecules (Basel, Switzerland), 25(14) (2020-07-19)
Cassava plays a key role in the food production and economies of several countries worldwide. Due to its starch content, alcoholic fermentation is a promising transformation process for adding value to cassava. However, most of the existing cassava beverages are
Isabel Pardo et al.
Biotechnology and bioengineering, 109(12), 2978-2986 (2012-06-26)
DNA recombination methods are useful tools to generate diversity in directed evolution protein engineering studies. We have designed an array of chimeric laccases with high-redox potential by in vitro and in vivo DNA recombination of two fungal laccases (from Pycnoporus
Rosario Díaz et al.
Applied microbiology and biotechnology, 88(1), 133-142 (2010-07-08)
Two laccase isoenzymes were purified and characterized from the basidiomycete Coriolopsis rigida during transformation of the water-soluble fraction of "alpeorujo" (WSFA), a solid residue derived from the olive oil production containing high levels of toxic compounds. Zymogram assays of laccases
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service