A24001
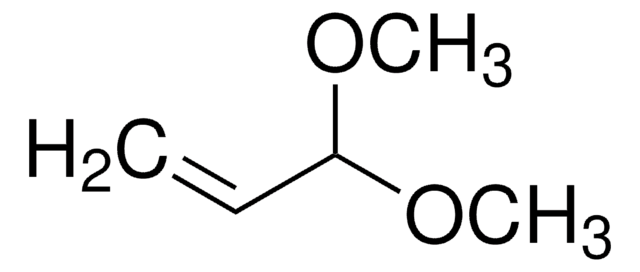
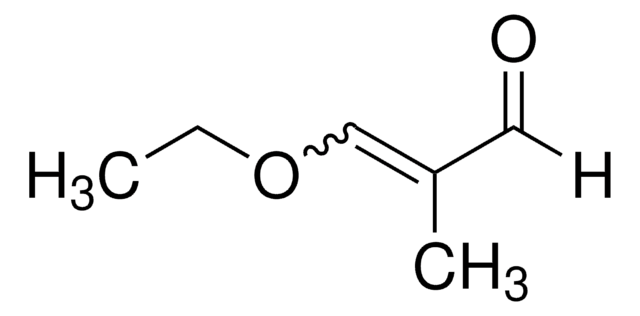
Acrolein diethyl acetal
96%
Synonym(s):
3,3-Diethoxy-1-propene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH2=CHCH(OCH2CH3)2
CAS Number:
Molecular Weight:
130.18
Beilstein:
1701567
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
form
liquid
refractive index
n20/D 1.398 (lit.)
bp
125 °C (lit.)
density
0.854 g/mL at 25 °C (lit.)
SMILES string
CCOC(OCC)C=C
InChI
1S/C7H14O2/c1-4-7(8-5-2)9-6-3/h4,7H,1,5-6H2,2-3H3
InChI key
MCIPQLOKVXSHTD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Acrolein diethyl acetal is widely used to carry out chemoselective Heck arylation to synthesize either 3-arylpropanoate esters or cinnamaldehyde derivatives.
It can also be used as one of the precursors to synthesize natural products like (−)-(Z)-Deoxypukalide, (−)-Laulimalide, botryodiplodin, neolaulimalide and isolaulimalide.
It can also be used as one of the precursors to synthesize natural products like (−)-(Z)-Deoxypukalide, (−)-Laulimalide, botryodiplodin, neolaulimalide and isolaulimalide.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
59.0 °F - closed cup
Flash Point(C)
15 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of (?)-and (−)-botryodiplodin using stereoselective radical cyclizations of acyclic esters and acetals.
Nouguier R et al.
Tetrahedron Asymmetry, 14(19), 3005-3018 (2003)
Total synthesis of neolaulimalide and isolaulimalide.
Gollner A and Mulzer J.
Organic Letters, 10(20), 4701-4704 (2008)
Chemoselective Heck arylation of acrolein diethyl acetal catalyzed by an oxime-derived palladacycle.
Najera C and Botella L
Tetrahedron, 61(41), 9688-9695 (2005)
Total Synthesis of Microtubule-Stabilizing Agent (-)-Laulimalide1.
Ghosh AK et al.
The Journal of Organic Chemistry, 66(26), 8973-8982 (2001)
An efficient palladium-catalyzed synthesis of cinnamaldehydes from acrolein diethyl acetal and aryl iodides and bromides.
Battistuzzi G, et al.
Organic Letters, 5(5), 777-780 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service