742724
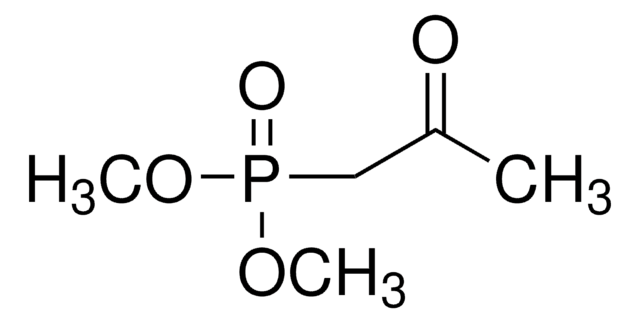
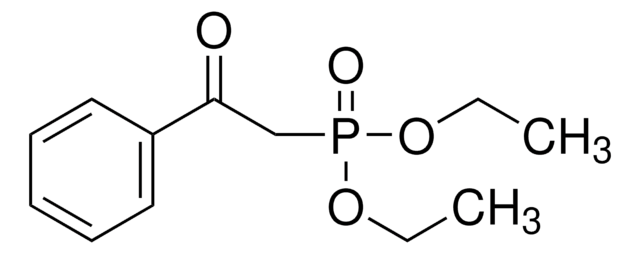
Dimethyl (1-diazo-2-oxopropyl)phosphonate solution
~10% in acetonitrile (H-NMR), ≥96% (HPLC)
Synonym(s):
Bestmann-Ohira Reagent, Dimethyl (1-azoacetonyl)phosphonate solution, Dimethyl (acetyldiazomethyl)phosphonate solution
About This Item
Recommended Products
Assay
≥96% (HPLC)
form
liquid
reaction suitability
reaction type: C-C Bond Formation
concentration
~10% in acetonitrile (H-NMR)
refractive index
n20/D 1.352-1.354
density
0.800-0.850 g/mL at 20 °C
functional group
ketone
phosphonate
storage temp.
2-8°C
SMILES string
COP(=O)(OC)C(=[N+]=[N-])C(C)=O
InChI
1S/C5H9N2O4P/c1-4(8)5(7-6)12(9,10-2)11-3/h1-3H3
InChI key
SQHSJJGGWYIFCD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Ethynyl compounds (alkynes) from aldehydes.
- Isoquinoline and pyridine N-oxides by cyclization with oximes in the presence of Rh catalyst.
- Dimethyl(diazomethyl)phosphonate through methanolysis, which is further employed as a reagent in the synthesis of enol ethers or alkynes.
- Five-member heterocyclic scaffolds of pyrazoles, triazoles, and oxazoles via cycloaddition reaction and multicomponent reaction.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
35.6 °F
Flash Point(C)
2 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Protocols
Acetylene chemistry has been and remains an important constituent element of molecular sciences. Its potential and widespread applications extend from organic synthesis through materials science to bioorganic chemistry. Some examples are enediynes (DNA-cleaving agents), ‘click chemistry’ tools and building blocks. Consequently, it triggers a demand for efficient syntheses of alkynes.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service