713031
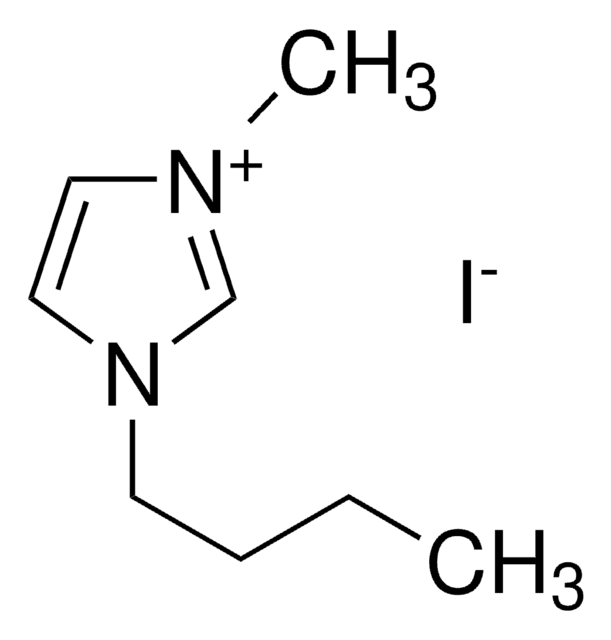
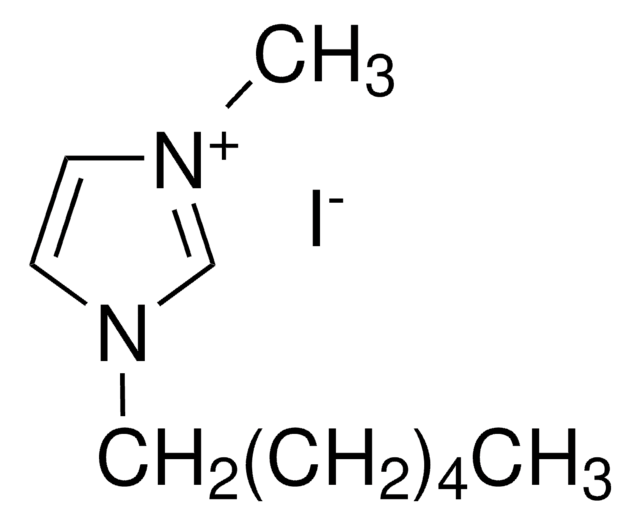
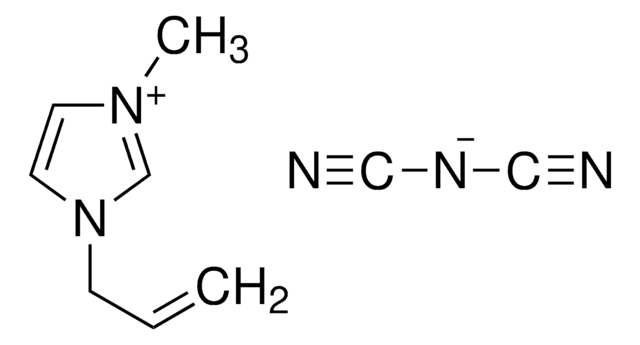
1-Ethyl-3-methylimidazolium iodide
97%
Synonym(s):
EMIMI
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H11IN2
CAS Number:
Molecular Weight:
238.07
Beilstein:
5160333
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥96.5% (HPLC)
97%
form
powder
impurities
≤0.5% water
SMILES string
[I-].CCn1cc[n+](C)c1
InChI
1S/C6H11N2.HI/c1-3-8-5-4-7(2)6-8;/h4-6H,3H2,1-2H3;1H/q+1;/p-1
InChI key
IKQCDTXBZKMPBB-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
1-Ethyl-3-methylimidazolium iodide is an ionic liquid that can be prepared by reacting methylimidazole with iodoethane. The addition of EMImI to 1-ethyl-3-methylimidazolium tetrafluoroborate (EMImBF4), increase its capacitance while developing electric double-layer capacitors (EDLCs) based on EMImBF4.
Application
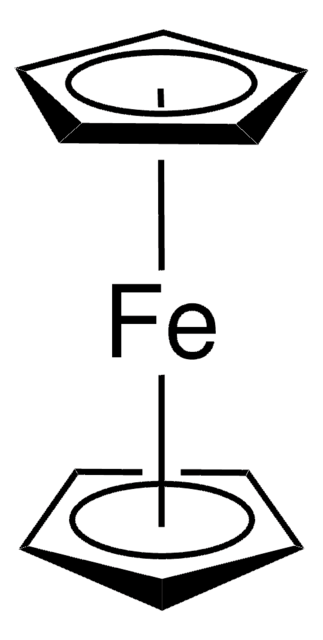
EMImI reacts with aluminum chloride to form 1-ethyl-3-methylimidazolium halogenoaluminate ionic liquid, [emim]I–(AlCl3)x, which is an excellent solvent for Friedel–Crafts acylation of ferrocene.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rong Chen et al.
Journal of colloid and interface science, 532, 727-737 (2018-08-20)
Novel Carbon quantum dots modified Bi5O7I (CQDs/Bi5O7I) nanorod composites were prepared via an ionic liquid 1-ethyl-3-methylimidazolium iodide ([Emim]I) assisted solvothermal method for the first time. Series of characterization methods were used to explore the structural composition, morphology and optical properties
Electrochemical Properties of Bi (111)| 1-Ethyl-3-Methylimidazolium Tetracyanoborate and 1-Ethyl-3-Methylimidazolium Iodide Interface
Simenson, Carolin, Liis Siinor, and Enn Lust
Journal of the Electrochemical Society, 30 (2015)
1-Ethyl-3-methylimidazolium halogenoaluminate ionic liquids as reaction media for the acylative cleavage of ethers
Green, Laine, Ivan Hemeon, and Robert D. Singer
Tetrahedron Letters, 41.9, 1343-1346 (2000)
1-Ethyl-3-methylimidazolium halogenoaluminate melts as reaction media for the Friedel?Crafts acylation of ferrocene
Jacqueline, K. D.
Chemical Communications (Cambridge, England), 24, 2753-2754 (1996)
Characteristics of Capacitors Based on Ionic Liquids: From Dielectric Polymers to Redox-Active Adsorbed Species.
Lust E, et al.
ECS Transactions, 75(15), 161-170 (2016)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service