All Photos(1)
About This Item
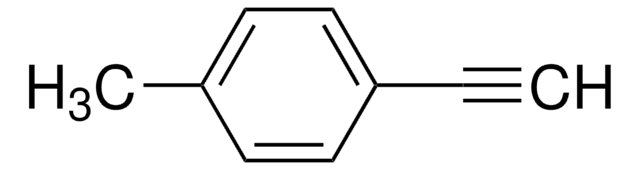
Linear Formula:
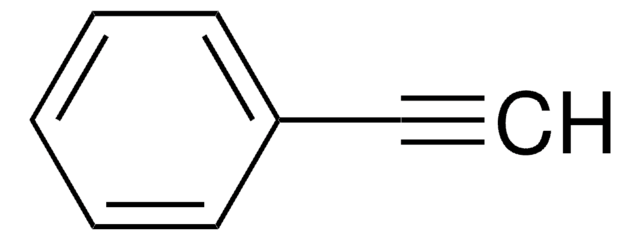
C6H5C6H4C≡CH
CAS Number:
Molecular Weight:
178.23
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
88-91 °C (lit.)
functional group
phenyl
SMILES string
C#Cc1ccc(cc1)-c2ccccc2
InChI
1S/C14H10/c1-2-12-8-10-14(11-9-12)13-6-4-3-5-7-13/h1,3-11H
InChI key
BPBNKCIVWFCMJY-UHFFFAOYSA-N
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Cu-Promoted Oxidative Trifluoromethylation of Terminal Alkynes with Difluoromethylene Phosphobetaine.
Deng X, et al.
Chin. J. Chem., 32(8), 689-693 (2014)
Hitoshi Fukuda et al.
The Japanese journal of antibiotics, 55(3), 270-280 (2002-08-30)
Convulsant activity of pazufloxacin mesilate (PZFX mesilate), a new quinolone antibacterial agent for intravenous use, in combination with nonsteroidal anti-inflammatory drug (NSAID) was investigated in mice after intravenous or intracerebroventricular administration. Following results were obtained. 1. In combination with 4-biphenylacetic
Shift of the acetylenic hydrogen during chemical and enzymatic oxidation of the biphenylacetylene triple bond.
P R Ortiz de Montellano et al.
Archives of biochemistry and biophysics, 209(2), 710-712 (1981-07-01)
A Wade et al.
The Biochemical journal, 188(3), 867-872 (1980-06-15)
1. The metabolism of 4-ethynylbiphenyl has been studied in vitro with subcellular fractions of normal and induced rat liver, and rat intestinal microflora (caecal contents). 2. Oxidation was NADPH-dependent, was inhibited by CO and stimulated by pretreatment with phenobarbitone or
Rajneesh Misra et al.
Dalton transactions (Cambridge, England : 2003), 43(12), 4854-4861 (2014-02-01)
A series of meso arylethynyl BODIPYs (2a-2h) were designed and synthesized by the Pd-catalyzed Sonogashira cross-coupling reaction. The effects of the donor on the photophysical properties of the BODIPYs were explored. The DFT optimized structures and crystal structures show the
Articles
The terminal alkyne functionality has a wide range of applications including most recently the synthesis of spiropyran substituted 2,3-dicyanopyrazines and (±)-asteriscanolide, as well as conversion to enamines using resin-bound 2° amines.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service