493805
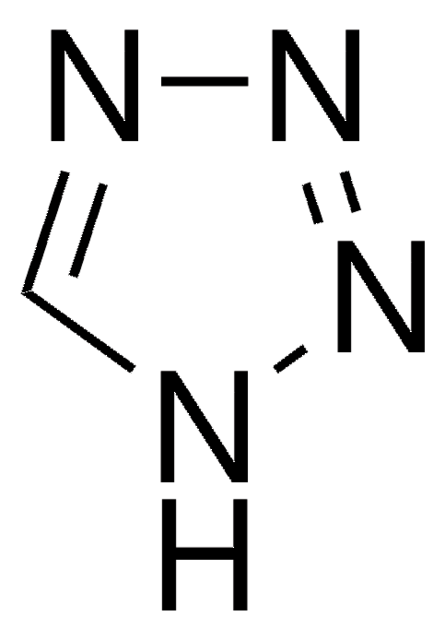
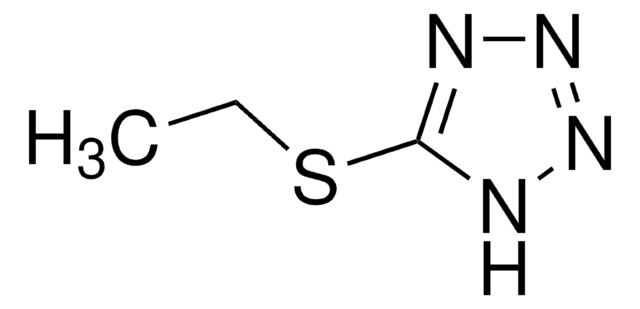
5-(Ethylthio)-1H-tetrazole
95%
Synonym(s):
5-(Ethylthio)-2H-tetrazole, 5-Ethylsulfanyl-2H-tetrazole
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C3H6N4S
CAS Number:
Molecular Weight:
130.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
mp
84-90 °C (lit.)
storage temp.
2-8°C
SMILES string
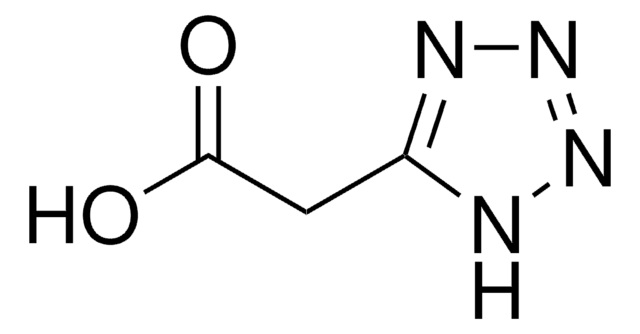
CCSc1nnn[nH]1
InChI
1S/C3H6N4S/c1-2-8-3-4-6-7-5-3/h2H2,1H3,(H,4,5,6,7)
InChI key
GONFBOIJNUKKST-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
5-(Ethylthio)-1H-tetrazole (ETT) is an efficient activator, that activates nucleoside phosphoramidites towards condensation with a nucleoside to form dinucleoside phosphates during oligonucleotide synthesis.
Application
- Solid-Phase Synthesis of Symmetrical Dinucleoside Phosphodiesters: Explores the efficiency of 5-(Ethylthio)-1H-tetrazole in the solid-phase synthesis of dinucleoside phosphodiesters, underlining its significance in the streamlined production of biologically relevant molecules (Ahmadibeni et al., 2007).
- Synthesis, Deprotection, Analysis, and Purification of RNA and Ribozymes: Utilizes 5-(Ethylthio)-1H-tetrazole in the synthesis and processing of RNA and ribozymes, highlighting its critical role in molecular biology and genetic engineering research (Wincott et al., 1995).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
4.1A - Other explosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
An efficient method for the isolation and purification of oligoribonucleotides.
Sproat B, et al.
Nucleosides, nucleotides & nucleic acids, 14(1-2), 255-273 (1995)
Gregor S Cremosnik et al.
Angewandte Chemie (International ed. in English), 53(1), 286-289 (2013-11-14)
P-Amidites can be used in iterative couplings to selectively give mixed P(III) -P(V) anhydrides. These intermediates can be oxidized followed by a rapid removal of the two terminal fluorenylmethyl groups. An iterative synthesis (coupling, oxidation, deprotection) of nucleoside oligophosphates can
Benzimidazolium Triflate as an Efficient Promoter for Nucleotide Synthesis via the Phosphoramidite Method.
Yoshihiro Hayakawa et al.
The Journal of organic chemistry, 61(23), 7996-7997 (1996-11-15)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service