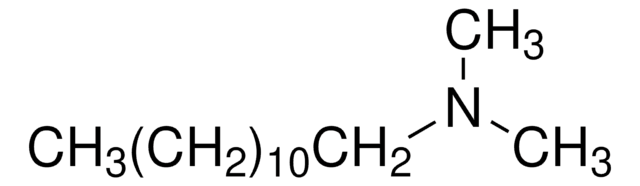All Photos(1)
About This Item
Linear Formula:
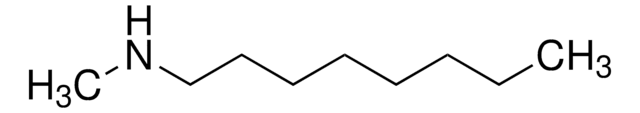
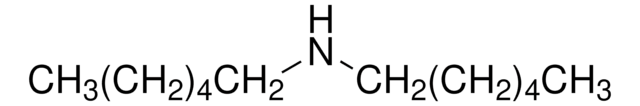
CH3(CH2)11NHCH3
CAS Number:
Molecular Weight:
199.38
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.44 (lit.)
bp
200 °C (lit.)
density
0.795 g/mL at 25 °C (lit.)
SMILES string
CCCCCCCCCCCCNC
InChI
1S/C13H29N/c1-3-4-5-6-7-8-9-10-11-12-13-14-2/h14H,3-13H2,1-2H3
InChI key
OMEMQVZNTDHENJ-UHFFFAOYSA-N
General description
N-Methyldodecylamine is a secondary fatty acid amine. It is formed during the pyrolysis of N-(2-cyanoethyl)-N-methyldodecylamine under reduced pressure.
Application
N-Methyldodecylamine may be used for the preparation of N,N,N,N,N,N-trimethyldodecylammonium bromide.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yongsoon Shin et al.
Journal of colloid and interface science, 284(1), 278-281 (2005-03-09)
We report here the self-assembly of surfactant molecules at the interface of air and the hygroscopic quaternary ammonium salt tetrabutylammonium acetate (TBAAc). Homogeneously dissolved surfactant molecules at 100 degrees C self-assemble upon contacting air due to high moisture adsorption by
Effect of the N-methylation of dodecylammonium chloride on the adsorption from its micellar solution
Aratono M, et al.
Bulletin of the Chemical Society of Japan, 61, 2773-2773 (1988)
B L Kabacoff et al.
IARC scientific publications, (57)(57), 347-352 (1984-01-01)
Nitrosation of water-soluble (diethanolamine) and oil-soluble (dodecylmethylamine and dicyclohexylamine) amines in the absence and presence of inhibitors in model anionic and non-ionic emulsions was studied. Nitrosation of diethanolamine occurred at similar rates in non-ionic and anionic emulsions. Surprisingly, dodecylmethylamine and
Kenichi Sakai et al.
Langmuir : the ACS journal of surfaces and colloids, 28(51), 17617-17622 (2012-11-20)
Rheological properties of alkyl dicarboxylic acid-alkylamine complex systems have been characterized. The complex materials employed in this study consist of an amino acid-based surfactant (dodecanoylglutamic acid, C12Glu) and a tertiary alkylamine (dodecyldimethylamine, C12DMA) or a secondary alkylamine (dodecylmethylamine, C12MA). (1)H
Wengang Liu et al.
The Science of the total environment, 702, 134593-134593 (2019-11-15)
With the extensive applications and ongoing world demand, more and more amine surfactants are discharged into natural environment. However, the database about toxicity of amine surfactants is incomplete, which is not beneficial to environmental protection process. In this paper, the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service