424471
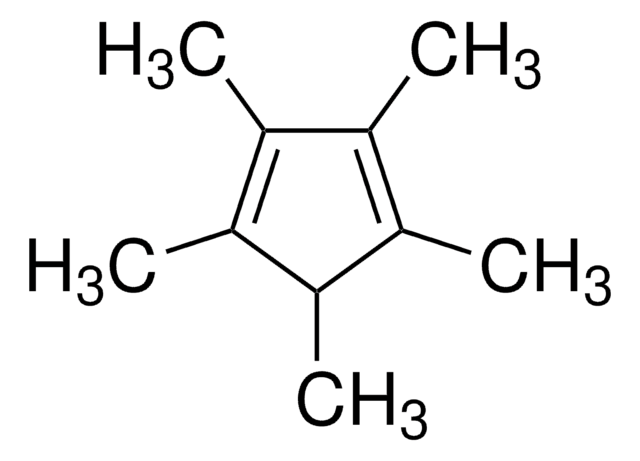
1,2,3,4-Tetramethyl-1,3-cyclopentadiene
~85%
Synonym(s):
1,2,3,4-Tetramethylcyclopentadiene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H14
CAS Number:
Molecular Weight:
122.21
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
~85%
form
liquid
refractive index
n20/D 1.472 (lit.)
bp
142 °C (lit.)
density
0.808 g/mL at 25 °C (lit.)
SMILES string
CC1=C(C)C(C)=C(C)C1
InChI
1S/C9H14/c1-6-5-7(2)9(4)8(6)3/h5H2,1-4H3
InChI key
VNPQQEYMXYCAEZ-UHFFFAOYSA-N
General description
1,2,3,4-Tetramethyl-1,3-cyclopentadiene is a tetra substituted 1,3-cyclopentadiene. It participates in the synthesis of resin-bound tetramethylcyclopentadienes.
Application
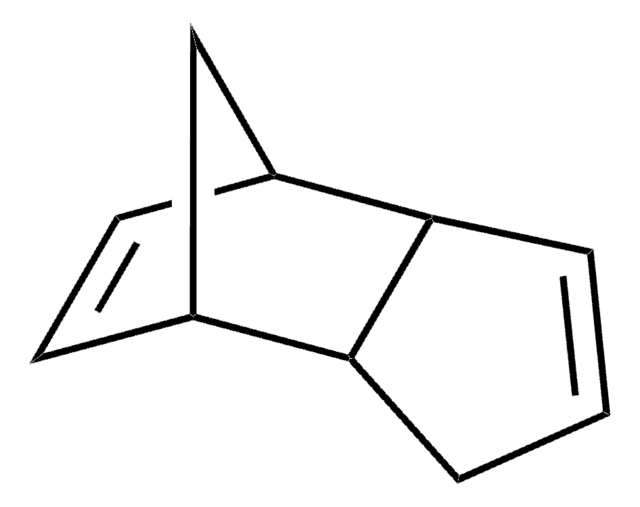
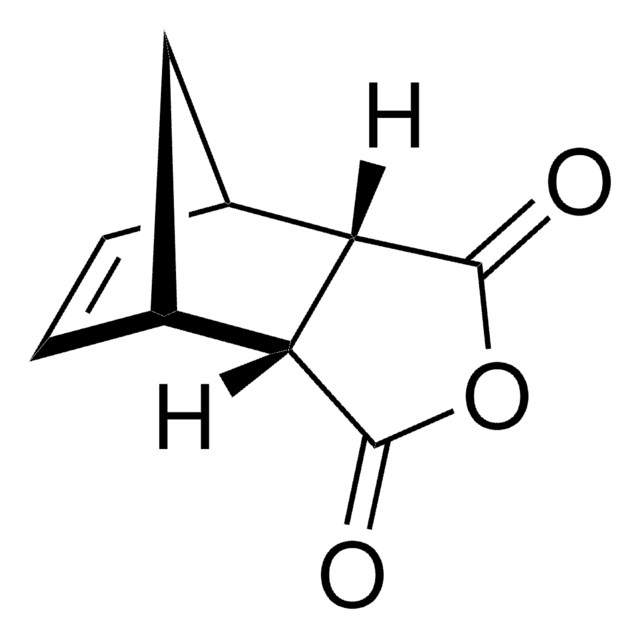
1,2,3,4-Tetramethyl-1,3-cyclopentadiene may be used as a starting material in the synthesis of 1,7,8,9-tetramethyl-4-oxa-tricyclo[5.2.1.02,6]dec-8-ene-3,5-dione.
Other Notes
remainder mixture of isomers
Signal Word
Warning
Hazard Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
107.6 °F - closed cup
Flash Point(C)
42 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of 4-Amino-1, 7, 8, 9-tetramethyl-4-aza-tricyclo [5.2. 1.02,6] dec-8-ene-3, 5-dione.
Struga M and Kossakowski J.
Molbank, 2007(2), M534-M534 (2007)
Selvan Demir et al.
Nature communications, 8(1), 2144-2144 (2017-12-17)
Increasing the operating temperatures of single-molecule magnets-molecules that can retain magnetic polarization in the absence of an applied field-has potential implications toward information storage and computing, and may also inform the development of new bulk magnets. Progress toward these goals
The synthesis of resin-bound tetramethylcyclopentadienes: an evaluation of two methodologies.
Shearer AS and de Miguel YR.
Tetrahedron Letters, 47(4), 447-450 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service