419702
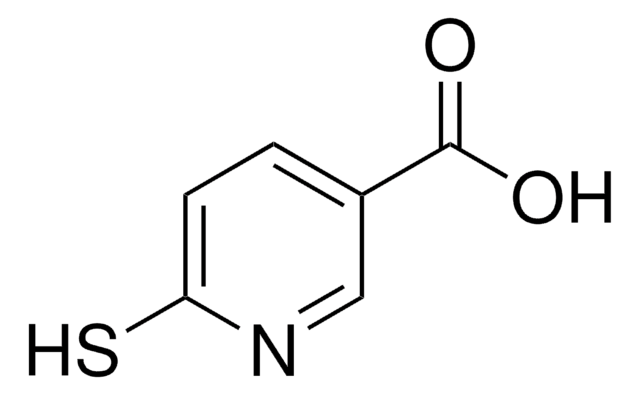
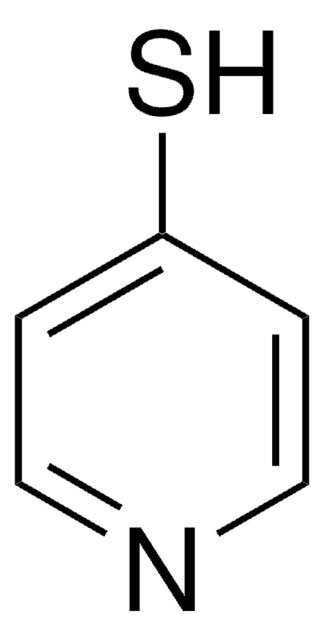
2-Mercaptopyridine-3-carboxylic acid
technical grade
Synonym(s):
2-Mercaptonicotinic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C6H5NO2S
CAS Number:
Molecular Weight:
155.17
Beilstein:
119029
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
form
powder
mp
263-265 °C (lit.)
functional group
carboxylic acid
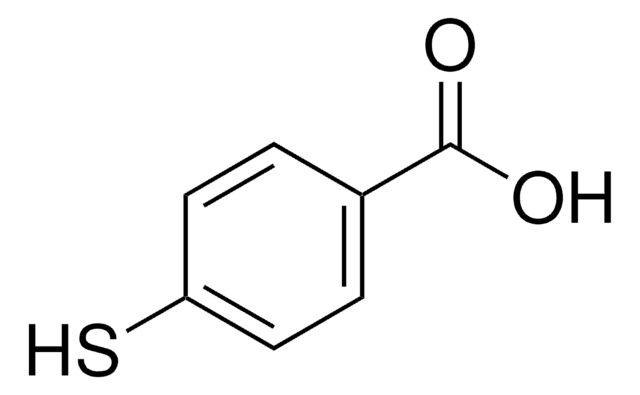
SMILES string
OC(=O)c1cccnc1S
InChI
1S/C6H5NO2S/c8-6(9)4-2-1-3-7-5(4)10/h1-3H,(H,7,10)(H,8,9)
InChI key
WYKHFQKONWMWQM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
2-Mercaptopyridine-3-carboxylic acid may be used for the synthesis of poly[[diaquabis([μ]2-4,4 ′-bipyridyl)iron(II)] bis{2-[(3-carboxypyridin-2-yl)disulfanyl]nicotinate}] and 2-(2-carboxyethylthio)nicotinic acid.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Aamir Jalil et al.
Molecular pharmaceutics, 15(8), 3527-3534 (2018-07-27)
The aim of this study was to synthesize iodine containing polymeric excipients for mucosal treatment of microbial infection exhibiting a prolonged mucosal residence time by forming an adhesive gel on the mucosal surface. In order to achieve this aim, 2-(2
Xiao-Juan Wang et al.
Acta crystallographica. Section E, Structure reports online, 66(Pt 6), o1298-o1298 (2010-01-01)
The title compound, C(8)H(7)NO(4)S·H(2)O, was obtained by reaction of 2-mercaptopyridine-3-carboxylic acid with chloro-acetic acid. In the mol-ecular structure, the dihedral angle between the two least-squares planes defined by the pyridine ring and the carb-oxy group is 8.32 (9)°. The carboxy-methyl-sulfanyl group
Alexandra Partenhauser et al.
Journal of biomedical materials research. Part B, Applied biomaterials, 105(3), 551-559 (2015-11-28)
Assessment of preactivated and thiolated silicone oils as potential long-term vitreous replacement. Thioglycolic acid (TGA) and 3-mercaptopropionic acid (MPA) were covalently coupled to amino-modified silicone oil and subsequently preactivated with 2-mercaptonicotinic acid (2-MNA). Each silicone thiomer was evaluated in view
Ioannis I Verginadis et al.
European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 42(3), 253-261 (2010-12-07)
Nowadays, investigation for possible therapeutic applications of various metal-based drugs attracts the scientific interest worldwide. The triorganotin compound bis[triphenyltin(IV)](3-carboxy-pyridine-2-thionato) (SnMNA), was tested for its anti-proliferative and antitumor activities. Cytotoxic activity was assessed by Trypan blue and 3-(4.5-dimethylthiazol-2-yl)-2.5-diphenyltetrazolium bromide assay (MTT).
A M Bode et al.
Biochemistry and cell biology = Biochimie et biologie cellulaire, 71(3-4), 113-121 (1993-03-01)
3-Mercaptopicolinae (3-MP) blocks gluconeogenesis from lactate, pyruvate, alanine, and other substrates through its inhibition of phosphoenolpyruvate carboxykinase. Nevertheless, we observed increased glycogenesis, net glucose uptake, and glucose-6-P levels in livers perfused with glucose in the presence of 3-MP. In perfusions
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service