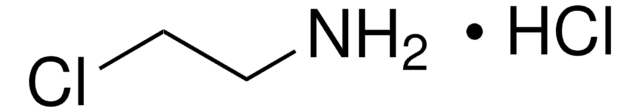
397261
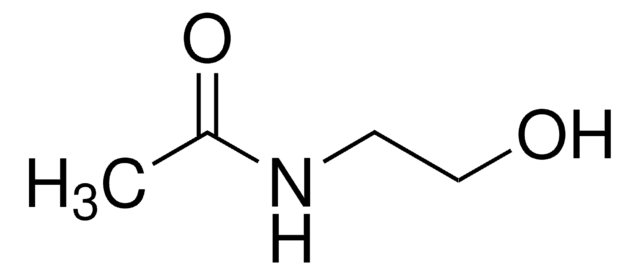
N-(2-Aminoethyl)acetamide
technical grade, 90%
Synonym(s):
N-Acetylethylenediamine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3C(O)NHCH2CH2NH2
CAS Number:
Molecular Weight:
102.14
Beilstein:
1743120
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
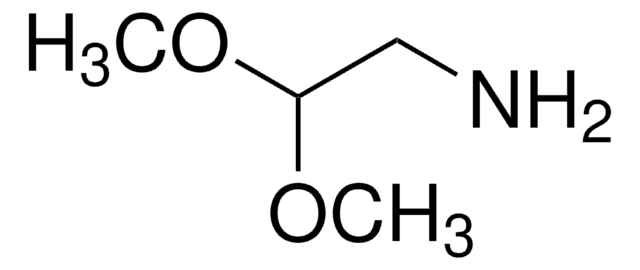
Recommended Products
grade
technical grade
Assay
90%
refractive index
n20/D 1.485 (lit.)
bp
128 °C/3 mmHg (lit.)
mp
50 °C (lit.)
density
1.066 g/mL at 25 °C (lit.)
functional group
amide
amine
SMILES string
CC(=O)NCCN
InChI
1S/C4H10N2O/c1-4(7)6-3-2-5/h2-3,5H2,1H3,(H,6,7)
InChI key
DAKZISABEDGGSV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
N-(2-Aminoethyl)acetamide is an organic building block.
Application
N-(2-Aminoethyl)acetamide may be used in the preparation of mixed two-component monolayers on glassy carbon. It may be used in the synthesis of lysidine.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
>230.0 °F - closed cup
Flash Point(C)
> 110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Enrique Huang Kwan et al.
Dalton transactions (Cambridge, England : 2003), 45(40), 15931-15941 (2016-07-08)
A new long-tethered boron-containing (P-B-P)-pincer ligand 8 has been synthesized. Complexation of 8 with [Ir(coe)
Emma J Wright et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 20(19), 5550-5554 (2014-04-12)
Mixed two-component monolayers on glassy carbon are prepared by electrochemical oxidation of N-(2-aminoethyl)acetamide and mono-N-Boc-hexamethylenediamine in mixed solution. Subsequent N-deprotection, amide coupling and solid-phase synthetic steps lead to electrode-surface functionalisation with maleimide, with controlled partial coverage of this cysteine-binding group
Yoonkyung Kim et al.
Purinergic signalling, 5(1), 39-50 (2008-07-05)
As a continued effort to develop multivalent ligands to enhance the pharmacological effects of monomeric drugs, DITC-APEC, a chemically reactive nucleoside A(2A) adenosine receptor (AR) agonist, was employed to derivatize the surface of third-generation (G3) polyamidoamine (PAMAM) dendrimers. The resulting
The Mechanism of Acid Hydrolysis of Lysidine and N-(2-Aminotheyl) acetamide and Related Amides.
Haake P and Watson J.
The Journal of Organic Chemistry, 35(12), 4063-4067 (1970)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service