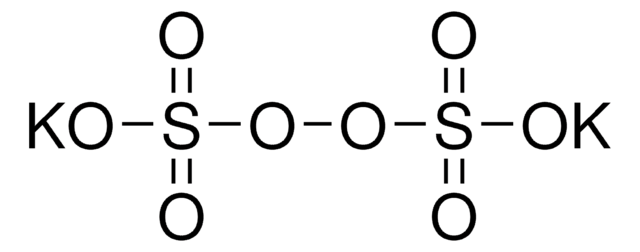379824
Potassium persulfate
99.99% trace metals basis
Synonym(s):
Potassium peroxodisulfate
About This Item
Recommended Products
vapor density
9.3 (vs air)
Quality Level
Assay
99.99% trace metals basis
form
solid
reaction suitability
reagent type: oxidant
impurities
≤150.0 ppm Trace Metal Analysis
SMILES string
[K+].[K+].[O-]S(=O)(=O)OOS([O-])(=O)=O
InChI
1S/2K.H2O8S2/c;;1-9(2,3)7-8-10(4,5)6/h;;(H,1,2,3)(H,4,5,6)/q2*+1;/p-2
InChI key
USHAGKDGDHPEEY-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Application
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Ox. Sol. 3 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
We presents an article regarding common FAQ's for initiators and stabalizers
Protocols
Monodisperse, surfactant-free polymer spheres for use as colloidal crystal templates can be easily obtained in reasonably large quantities. Typical synthesis methods for poly(methyl methacrylate) (PMMA) and poly(styrene) (PS) by emulsifier free emulsion polymerization are described below and yield spheres several hundred nanometers in diameter.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service