All Photos(1)
About This Item
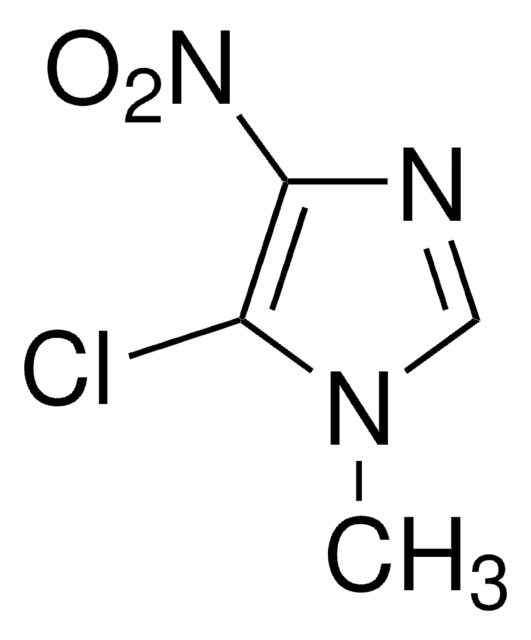
Empirical Formula (Hill Notation):
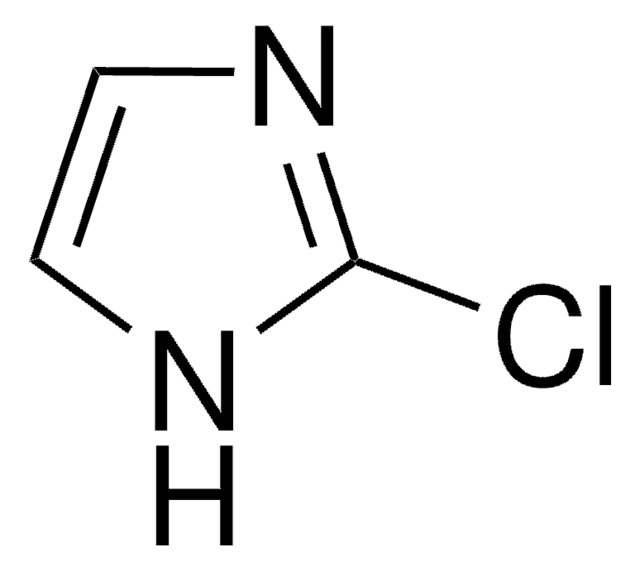
C4H5ClN2
CAS Number:
Molecular Weight:
116.55
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.511 (lit.)
bp
82-85 °C/11 mmHg (lit.)
density
1.25 g/mL at 25 °C (lit.)
functional group
chloro
SMILES string
Cn1cncc1Cl
InChI
1S/C4H5ClN2/c1-7-3-6-2-4(7)5/h2-3H,1H3
InChI key
NYDGOZPYEABERA-UHFFFAOYSA-N
General description
5-Chloro-1-methylimidazole is a 5-halo-1-methylimidazole. It participates in electron-rich iron(III) porphyrin complex catalyzed epoxidation of olefins.
Application
5-Chloro-1-methylimidazole was used in synthesis of C-5 functionalized N-methylated imidazoles.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
204.8 °F - closed cup
Flash Point(C)
96 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
An efficient route to 5-iodo-1-methylimidazole: synthesis of xestomanzamine A.
Panosyan FB and Still IWJ.
Canadian Journal of Chemistry, 79(7), 1110-1114 (2001)
W Nam et al.
Journal of inorganic biochemistry, 80(3-4), 219-225 (2000-09-23)
An electron-rich iron(III) porphyrin complex (meso-tetramesitylporphinato)iron(III) chloride [Fe(TMP)Cl], was found to catalyze the epoxidation of olefins by aqueous 30% H2O2 when the reaction was carried out in the presence of 5-chloro-1-methylimidazole (5-Cl-1-Melm) in aprotic solvent. Epoxides were the predominant products
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service