303836
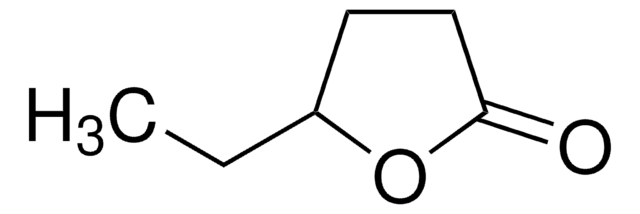
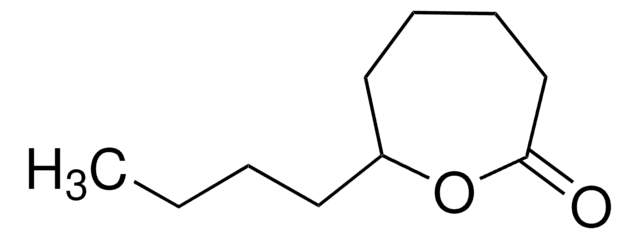
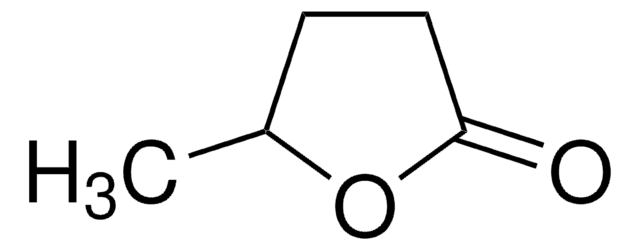
γ-Caprolactone
98%
Synonym(s):
gamma-Caprolactone, γ-Ethyl-γ-butyrolactone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H10O2
CAS Number:
Molecular Weight:
114.14
Beilstein:
6114822
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.439 (lit.)
bp
219 °C (lit.)
density
1.023 g/mL at 25 °C (lit.)
functional group
ester
SMILES string
CCC1CCC(=O)O1
InChI
1S/C6H10O2/c1-2-5-3-4-6(7)8-5/h5H,2-4H2,1H3
InChI key
JBFHTYHTHYHCDJ-UHFFFAOYSA-N
Gene Information
human ... CYP1A2(1544)
Looking for similar products? Visit Product Comparison Guide
General description
γ-caprolactone (GCL) is well-known as a food flavor and is a biostimulant molecule promoting the growth of bacteria with biocontrol activity against soft-rot pathogens. GCL treatment stimulates growth of the native quorum sensing-degrading bacterial community in an industrial plant hydroponic system for culturing Solanum tuberosum.
Application
γ-Caprolactone was used as curing agent for diglycidyl ether of bisphenol A with ytterbium triflate as an initiator.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
208.4 °F - closed cup
Flash Point(C)
98 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Corinne Barbey et al.
Journal of proteome research, 11(1), 206-216 (2011-11-17)
Gamma-caprolactone (GCL) is well-known as a food flavor and has been recently described as a biostimulant molecule promoting the growth of bacteria with biocontrol activity against soft-rot pathogens. Among these biocontrol agents, Rhodococcus erythropolis, characterized by a remarkable metabolic versatility
Amélie Cirou et al.
Research in microbiology, 162(9), 945-950 (2011-02-04)
Bacteria degrading quorum sensing (QS) signals have been proposed as biocontrol agents able to quench QS-dependent expression of virulence symptoms caused by Pectobacterium on potato plants. We report here that gamma-caprolactone (GCL) treatment stimulated growth of the native QS-degrading bacterial
New thermosets obtained by the cationic copolymerization of diglycidyl ether of bisphenol A with ?-caprolactone with an improvement in the shrinkage. I. Study of the chemical processes and physical characteristics.
Gonzalez S, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 45(10), 1968-1979 (2007)
Olga Khersonsky et al.
Biochemistry, 44(16), 6371-6382 (2005-04-20)
PON1 is the best-studied member of a family of enzymes called serum paraoxonases, or PONs, identified in mammals (including humans) and other vertebrates as well as in invertebrates. PONs exhibit a range of important activities, including drug metabolism and detoxification
H Thoma et al.
Journal of chromatography, 309(1), 17-32 (1984-07-13)
A two-step ultrafiltration method combined with anion-exchange chromatography is described for the separation of lipophilic and strongly lipophobic acids occurring in human blood, serum and plasma. After treatment with diazomethane, the acid fractions are separated further by gas chromatography. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service