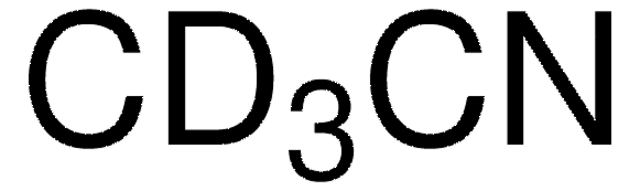296147
Dimethyl sulfoxide-d6
99.9 atom % D, contains 0.03 % (v/v) TMS
Synonym(s):
(Methyl sulfoxide)-d6, DMSO-d6, Hexadeuterodimethyl sulfoxide
About This Item
Recommended Products
vapor pressure
0.42 mmHg ( 20 °C)
Quality Level
isotopic purity
99.9 atom % D
Assay
99% (CP)
form
liquid
autoignition temp.
573 °F
contains
0.03 % (v/v) TMS
expl. lim.
42 %
technique(s)
NMR: suitable
impurities
≤0.0250% water
water
refractive index
n20/D 1.476 (lit.)
bp
189 °C (lit.)
mp
20.2 °C (lit.)
density
1.190 g/mL at 25 °C (lit.)
mass shift
M+6
SMILES string
[2H]C([2H])([2H])S(=O)C([2H])([2H])[2H]
InChI
1S/C2H6OS/c1-4(2)3/h1-2H3/i1D3,2D3
InChI key
IAZDPXIOMUYVGZ-WFGJKAKNSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Estimation of dissociation constants (pKa′s) of oximes from proton chemical shifts in dimethyl sulfoxide solution: This research utilizes Dimethyl sulfoxide-d₆ (DMSO-d₆) to determine pKa values of oximes, demonstrating its crucial role in enhancing the accuracy of proton NMR spectroscopy for chemical analysis and research within pharmaceutical contexts (Kurtz AP, D′Silva TD, 1987).
- Proton nuclear magnetic resonance studies on methylthiohydantoins, thiohydantoins, and hydantoins of amino acids: DMSO-d₆ is extensively employed to investigate the structure and behavior of various hydantoin derivatives in amino acid research, proving essential for understanding protein structures and functions, with implications for both academic and pharmaceutical industries (Suzuki T, Tomioka T, Tuzimura K, 1977).
Recommended products
accessory
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
190.4 °F
Flash Point(C)
88 °C
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
NMR spectroscopy elucidates molecular structure and purity via nuclear spin states in a strong magnetic field.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service