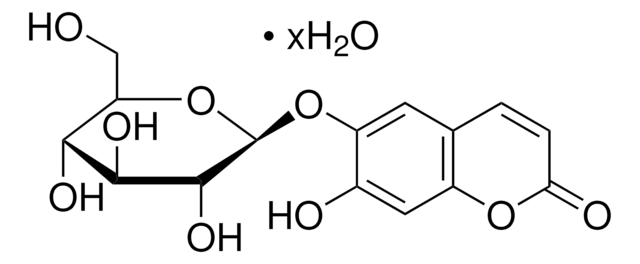
246573
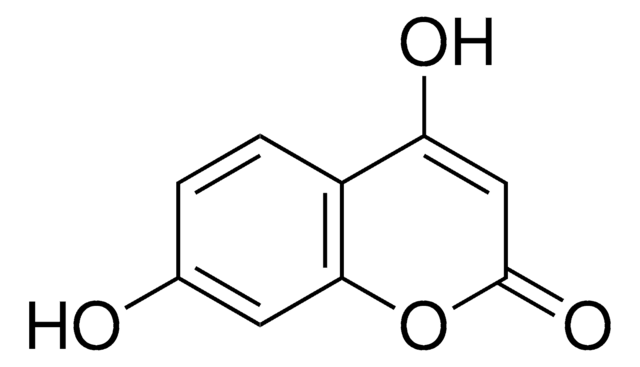
6,7-Dihydroxycoumarin
98%
Synonym(s):
Cichorigenin, Esculetin
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H6O4
CAS Number:
Molecular Weight:
178.14
Beilstein:
152788
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
98%
form
powder
mp
271-273 °C (lit.)
SMILES string
Oc1cc2OC(=O)C=Cc2cc1O
InChI
1S/C9H6O4/c10-6-3-5-1-2-9(12)13-8(5)4-7(6)11/h1-4,10-11H
InChI key
ILEDWLMCKZNDJK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chen Wang et al.
Journal of neurochemistry, 121(6), 1007-1013 (2012-03-31)
Previous studies have demonstrated that a natural coumarin compound esculetin (Esc) possesses antioxidant, anti-tumor, and anti-inflammation activities and rescues cultured primary neurons from NMDA toxicity. In this study, we investigated the neuroprotective effects of Esc on cerebral ischemia/reperfusion (I/R) injury
Aline Witaicenis et al.
Chemico-biological interactions, 186(2), 211-218 (2010-04-13)
Coumarins comprise a broad class of phenolic compounds that influences the formation and scavenging of reactive oxygen species and the processes involving free radical-mediated injury. In light of the antioxidant and anti-inflammatory properties of esculetin and 4-methylesculetin, the aim of
Sudhakar R Subramaniam et al.
Toxicology and applied pharmacology, 250(2), 130-136 (2010-10-12)
Esculetin (6,7-dihydroxy coumarin), is a potent antioxidant that is present in several plant species. The aim of this study was to investigate the mechanism of protection of esculetin in human hepatoma HepG2 cells against reactive oxygen species (ROS) induced by
Li-Wen Tian et al.
Journal of natural products, 72(6), 1057-1060 (2009-05-09)
Five new 7-O-methylkaempferol and -quercetin glycosides, namely, nervilifordins A-E (1-5), were isolated from the whole plant of Nervilia fordii, together with seven known flavonoids (6, 7, and 9-13) and one known coumarin (8). Their structures were elucidated on the basis
Eun-Sun Yun et al.
Toxicology in vitro : an international journal published in association with BIBRA, 25(7), 1335-1342 (2011-05-24)
The phenolic compound esculetin is known to inhibit the proliferation of vascular smooth muscle cells (VSMC). However, the signaling pathway by which esculetin mediates its molecular effects in VSMC remains to be identified. The present results suggest an unexpected role
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service