All Photos(1)
About This Item
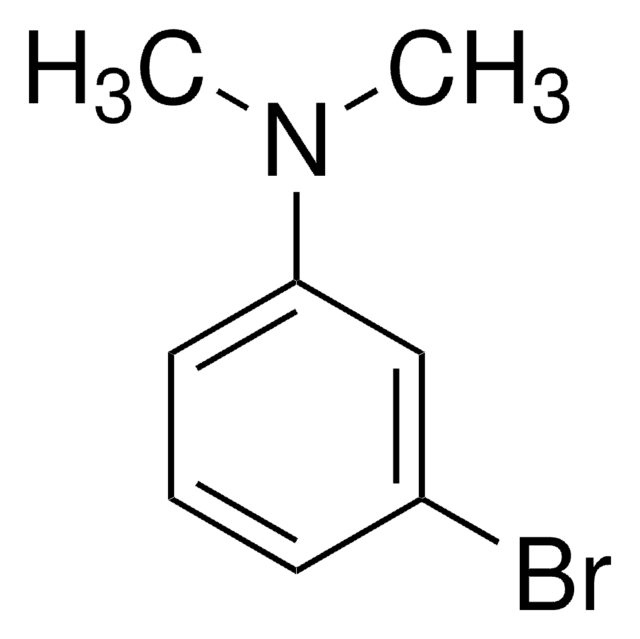
Linear Formula:
BrC6H4N(CH3)2
CAS Number:
Molecular Weight:
200.08
Beilstein:
2206155
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
bp
264 °C (lit.)
mp
52-54 °C (lit.)
functional group
amine
SMILES string
CN(C)c1ccc(Br)cc1
InChI
1S/C8H10BrN/c1-10(2)8-5-3-7(9)4-6-8/h3-6H,1-2H3
InChI key
XYZWMVYYUIMRIZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
4-Bromo-N,N-dimethylaniline was used as an internal standard in the determination of iodine present as iodide (as in pharmaceuticals), iodate (as in iodized table salt) and covalently bound to organic compounds (as in milk and vegetables).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Paramita Das et al.
Journal of chromatography. A, 1023(1), 33-39 (2004-02-06)
A rapid sequence of oxidation and iodination using 2-iodosobenzoate as an oxidizing agent and N,N-dimethylaniline as an iodine scavenger at pH 6.4, when 4-iodo-N,N-dimethylaniline is formed, has been used for the determination of iodide by GC-MS. Solid phase microextraction (SPME)
Jisheng Zhang et al.
Dalton transactions (Cambridge, England : 2003), 44(21), 9847-9859 (2015-05-06)
Utilization of dioxygen as the terminal oxidant at ambient temperature is always a challenge in redox chemistry, because it is hard to oxidize a stable redox metal ion like iron(III) to its high oxidation state to initialize the catalytic cycle.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service