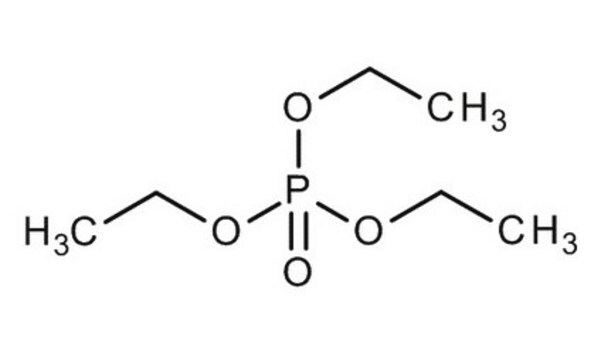
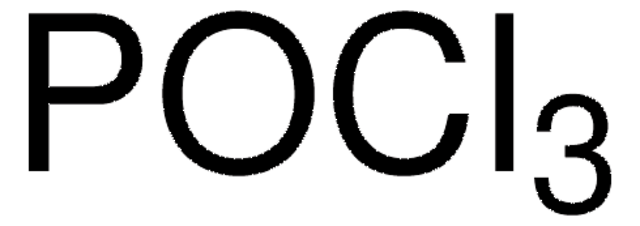241024
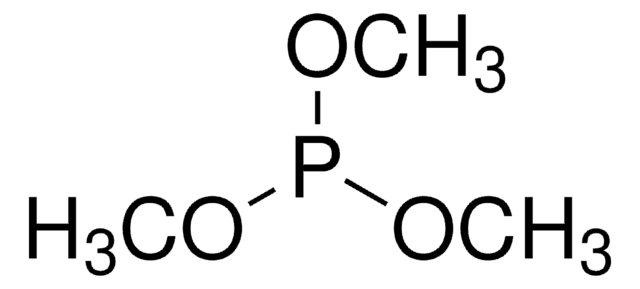
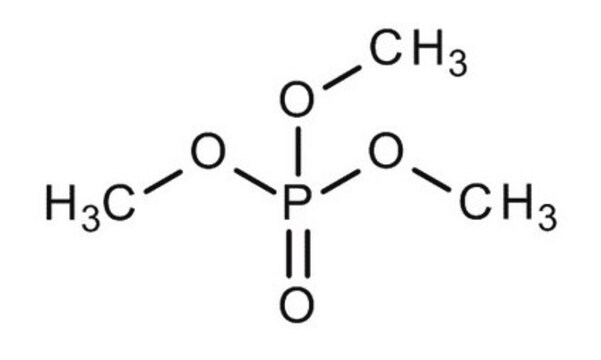
Trimethyl phosphate
≥99%
Synonym(s):
TMP, TMPA, TMPO
About This Item
Recommended Products
Quality Level
Assay
≥99%
form
liquid
refractive index
n20/D 1.395 (lit.)
bp
197 °C (lit.)
mp
−46 °C (lit.)
density
1.197 g/mL at 25 °C (lit.)
functional group
phosphate
SMILES string
COP(=O)(OC)OC
InChI
1S/C3H9O4P/c1-5-8(4,6-2)7-3/h1-3H3
InChI key
WVLBCYQITXONBZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Enhanced Battery Performance: Trimethyl phosphate is utilized to manipulate the ionic conductivity and interfacial compatibility in polymer-in-dual-salt electrolytes, significantly extending the operable temperature range of quasi-solid metal batteries (Lin et al., 2024).
- Thermochemical Properties Analysis: Research on trimethyl phosphate′s thermochemistry properties and rate kinetics, particularly H-Atom Abstraction reactions, provides valuable insights into its stability and reactivity, which are crucial for various chemical synthesis applications (Bruce and Li, 2023).
- Organophosphorus Flame Retardants Detection: Trimethyl phosphate is explored for its role in the screening of organophosphorus flame retardant residues in food samples, specifically rice, utilizing advanced chromatographic techniques to ensure food safety and compliance with environmental regulations (Li et al., 2023).
- Interfacial Reaction Regulation in Batteries: The study on regulating interfacial reactions through electrolyte chemistry, involving trimethyl phosphate, highlights its potential to create gradient interphases for low-temperature zinc metal batteries, contributing to enhanced battery safety and efficiency (Wang et al., 2023).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Muta. 1B - Skin Irrit. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 1
Flash Point(F)
302.0 °F - closed cup
Flash Point(C)
150 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service