All Photos(1)
About This Item
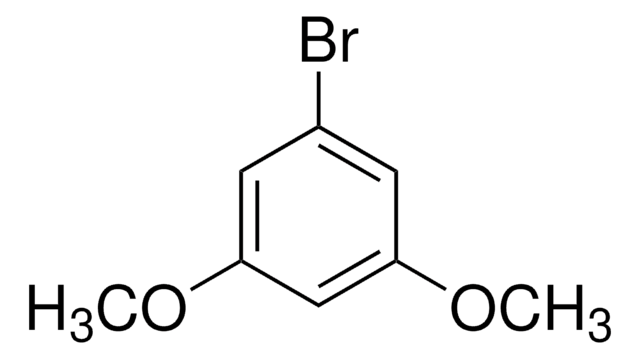
Linear Formula:
ClC6H3(OCH3)2
CAS Number:
Molecular Weight:
172.61
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
34-36 °C (lit.)
functional group
chloro
SMILES string
COc1cc(Cl)cc(OC)c1
InChI
1S/C8H9ClO2/c1-10-7-3-6(9)4-8(5-7)11-2/h3-5H,1-2H3
InChI key
WQHNWJBSROXROL-UHFFFAOYSA-N
General description
5-Chloro-1,3-dimethoxybenzene participates in Suzuki-Miyaura cross-coupling reactions of aryl- and heteroaryl chlorides with potassium cyclobutyltrifluoroborate.
Application
5-Chloro-1,3-dimethoxybenzene was used in the regioselective synthesis of TMC-264, tricyclic structure having chloro-1H-dibenzo[b,d]pyran-4,6-dione skeleton.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
233.6 °F - closed cup
Flash Point(C)
112 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
The first total synthesis and structural determination of TMC-264.
Tatsuta K, et al.
Tetrahedron Letters, 49(25), 4036-4039 (2008)
Gary A Molander et al.
The Journal of organic chemistry, 73(19), 7481-7485 (2008-09-02)
Suitable conditions were found for the Suzuki-Miyaura cross-coupling reaction of potassium cyclopropyl- and cyclobutyltrifluoroborates with aryl chlorides. Both of these secondary alkyl trifluoroborates coupled in moderate to excellent yield with electron-rich, electron-poor, and hindered aryl chlorides to give a variety
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service