All Photos(1)
About This Item
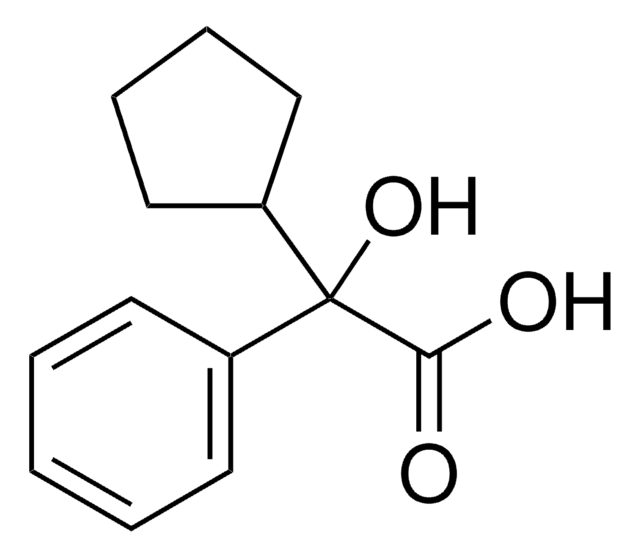
Linear Formula:
C5H9CH(C6H5)CO2H
CAS Number:
Molecular Weight:
204.26
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
98-100 °C (lit.)
solubility
methanol: soluble 50 mg/mL, clear, colorless
functional group
carboxylic acid
phenyl
SMILES string
OC(=O)C(C1CCCC1)c2ccccc2
InChI
1S/C13H16O2/c14-13(15)12(11-8-4-5-9-11)10-6-2-1-3-7-10/h1-3,6-7,11-12H,4-5,8-9H2,(H,14,15)
InChI key
BCJIDGDYYYBNNB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Cyclopentylphenylacetic acid was used in the synthesis of:
- 1-cyclopentyl-l-phenyl-2-(p-alkoxyphenyl)ethylenes
- soft ester analogs of anticholinergics
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
B Liebmann et al.
Arzneimittel-Forschung, 42(11), 1354-1358 (1992-11-01)
Ester hydrolysis represents an important biotransformation pathway for various parasympatholytic agents. Cleavage of the ciclotropium ester bond results in the formation of alpha-phenylciclopentylacetic acid (PCA). The relevance of this metabolic route for ciclotropium bromide (HIT-PCE, CAS 85166-20-7) including its stereochemical
F Huang et al.
Die Pharmazie, 57(2), 115-121 (2002-03-07)
Four new soft anticholinergic agents based on tropyl alpha-phenylcyclopentylacetate, 15a, 15b, 18a, and 18b, were designed and synthesized. Receptor binding studies on the cloned human muscarinic receptors indicated that the new soft anticholinergic agents possessed moderate potency as pKi ranged
N Bodor et al.
Journal of medicinal chemistry, 23(5), 474-480 (1980-05-01)
A new class of antimuscarinic drugs was designed and synthesized. The compounds are "soft" quaternary ammonium esters in which there is only one carbon atom separating the ester oxygen and the quaternary head. The compounds are potent anticholinergics when derived
664. Chemical constitution and sex-hormonal activity: the synthesis of of some 1-cyclopentyl-and 1-cyclohexyl-1: 2-diarylethylenes.
Hey DH and Musgrave OC.
Journal of the Chemical Society, 3156-3164 (1949)
Radoslaw Laufer et al.
Bioorganic & medicinal chemistry, 22(17), 4968-4997 (2014-07-22)
TTK kinase was identified by in-house siRNA screen and pursued as a tractable, novel target for cancer treatment. A screening campaign and systematic optimization, supported by computer modeling led to an indazole core with key sulfamoylphenyl and acetamido moieties at
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service