All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C12H12N2O
CAS Number:
Molecular Weight:
200.24
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
mp
92-94 °C (lit.)
functional group
phenyl
SMILES string
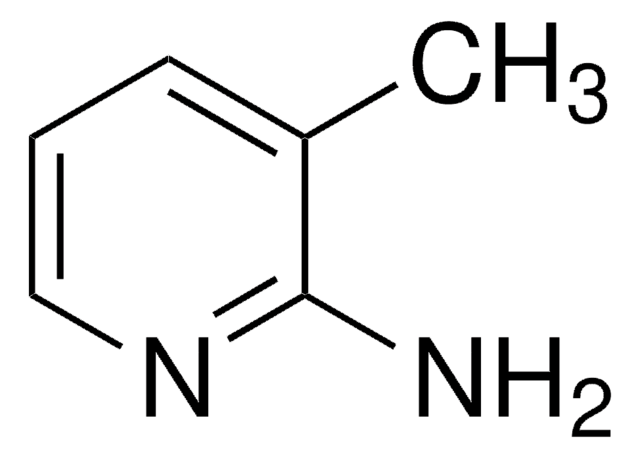
Nc1ncccc1OCc2ccccc2
InChI
1S/C12H12N2O/c13-12-11(7-4-8-14-12)15-9-10-5-2-1-3-6-10/h1-8H,9H2,(H2,13,14)
InChI key
NMCBWICNRJLKKM-UHFFFAOYSA-N
Gene Information
human ... MAPK14(1432)
General description
2-Amino-3-benzyloxypyridine on condensation with diethyl ethoxymethylene malonate affords 9-benzyloxy-3-ethoxycarbonylpyrido[1,2-a]pyrimidin-4-one.
Application
2-Amino-3-benzyloxypyridine was used in the synthesis of 1-acetyl-2-[2-(3-benzyloxypyridinyl)]iminoimidazolidine.
Biochem/physiol Actions
2-Amino-3-benzyloxypyridine is an inhibitor of mitogen-activated protein kinase p38α activity.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of new heterocyclic phenols: 9-Hydroxypyrido [1, 2-a] pyrimidin-4-one and Derivatives.
Dennin F, et al.
Journal of Heterocyclic Chemistry, 28(5), 1287-1291 (1991)
J Chang-Fong et al.
Chemical & pharmaceutical bulletin, 48(5), 729-733 (2000-05-24)
In order to obtain possible veinotonic drugs acting through alpha2 receptor activation, we prepared clonidine analogues in which the 2-imino-imidazolidine was attached to various aliphatic or aromatic heterocycles. Among them, the two benzopyranic derivatives 16 and 22 exhibited interesting affinities
Jung-Eun Park et al.
European journal of medicinal chemistry, 141, 322-334 (2017-10-17)
Herein, we report synthesis and evaluation of new twenty six small molecules against β amyloid (Aβ)-induced opening of mitochondrial permeability transition pore (mPTP) using JC-1 assay which measures the change of mitochondrial membrane potential (ΔΨm). The neuroprotective effect of seventeen
Ahmed Elkamhawy et al.
European journal of medicinal chemistry, 128, 56-69 (2017-02-06)
Herein, we report a new series of aliphatic substituted pyridyl-urea small molecules synthesized as potential modulators for amyloid beta (Aβ) induced mitochondrial dysfunction. Their blocking activities against Aβ-induced mitochondrial permeability transition pore (mPTP) opening were evaluated by JC-1 assay which
Yuya Kodama et al.
Journal of medicinal chemistry, 56(22), 9342-9350 (2013-11-01)
In this study, we developed an assignment-free approach for rapid identification of ligand-binding sites in target proteins by using NMR. With a sophisticated cell-free stable isotope-labeling procedure that introduces (15)N- or (13)C-labels to specific atoms of target proteins, this approach
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service