115282
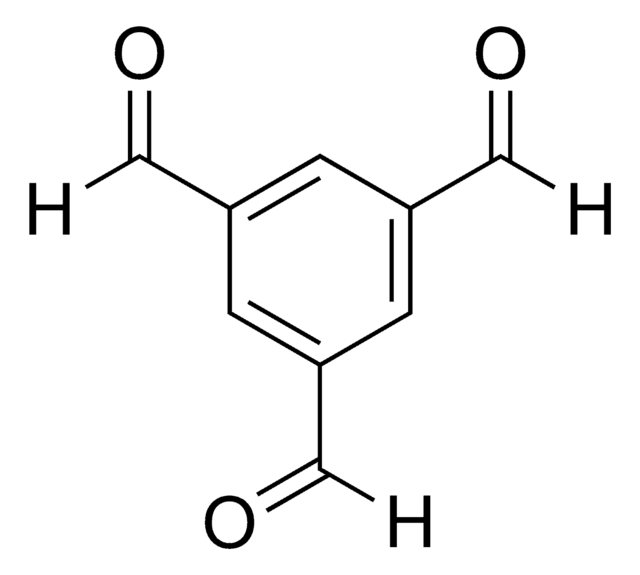
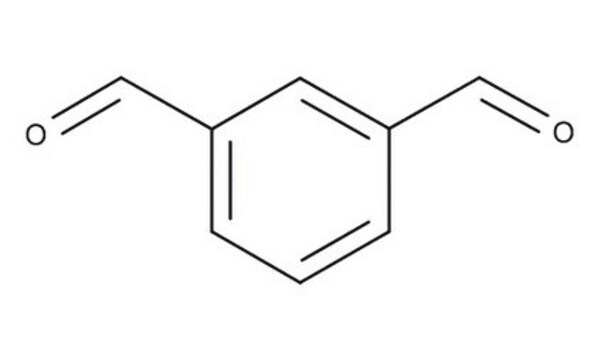
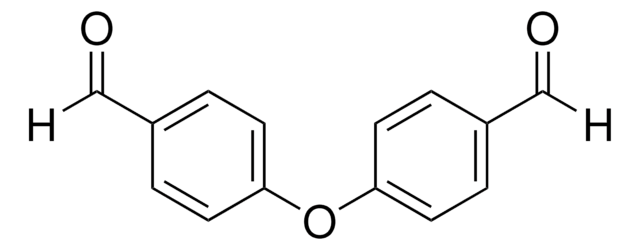
Isophthalaldehyde
97%
Synonym(s):
Benzene-1,3-dicarboxaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H4-1,3-(CHO)2
CAS Number:
Molecular Weight:
134.13
Beilstein:
1561038
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
87-88 °C (lit.)
SMILES string
O=Cc1cccc(C=O)c1
InChI
1S/C8H6O2/c9-5-7-2-1-3-8(4-7)6-10/h1-6H
InChI key
IZALUMVGBVKPJD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Isophthalaldehyde participates in base-catalyzed Knoevenagel condensation reaction.
Application
Isophthalaldehyde is used in the synthesis of binuclear ruthenium complex.
Packaging
Packaged in glass bottles
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Johanna Andersson et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 16(36), 11037-11046 (2010-08-04)
The binuclear ruthenium complex [μ-bidppz(phen)(4)Ru(2)](4+) has been extensively studied since the discovery of its unusual threading intercalation interaction with DNA, a binding mode with extremely slow binding and dissociation kinetics. The complex has been shown to be selective towards long
The effect of outer-sphere acidity on chemical reactivity in a synthetic heterogeneous base catalyst.
John D Bass et al.
Angewandte Chemie (International ed. in English), 42(42), 5219-5222 (2003-11-06)
Articles
Knoevenagel Condensation is an organic reaction named after Emil Knoevenagel. It is a classic C-C bond formation reaction and a modification of the Aldol Condensation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service