112402
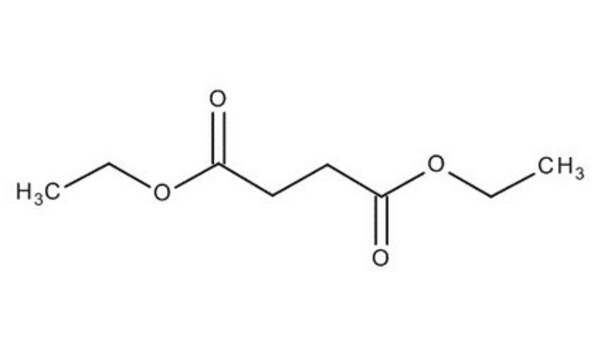
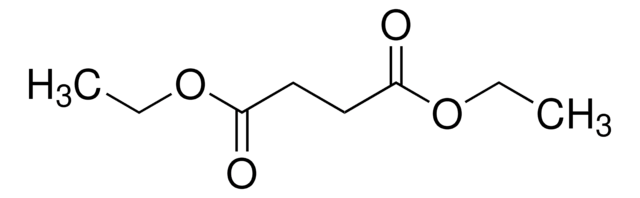
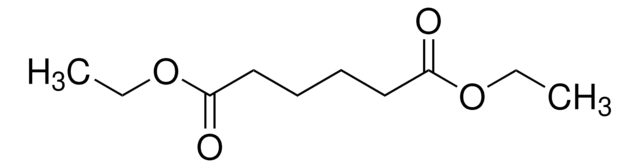
Diethyl succinate
ReagentPlus®, 99%
Synonym(s):
1,4-Diethyl butanedioate, Diethyl butanedioate, Ethyl succinate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C2H5OCOCH2CH2COOC2H5
CAS Number:
Molecular Weight:
174.19
Beilstein:
907645
EC Number:
MDL number:
UNSPSC Code:
12162002
eCl@ss:
39022839
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
vapor density
6 (vs air)
Quality Level
product line
ReagentPlus®
Assay
99%
form
liquid
refractive index
n20/D 1.42 (lit.)
bp
218 °C (lit.)
mp
−20 °C (lit.)
density
1.047 g/mL at 25 °C (lit.)
SMILES string
CCOC(=O)CCC(=O)OCC
InChI
1S/C8H14O4/c1-3-11-7(9)5-6-8(10)12-4-2/h3-6H2,1-2H3
InChI key
DKMROQRQHGEIOW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Diethyl succinate(DES) is a diethyl ester with succinate molecules. It has two ester groups and is majorly used in fragrances. It produced by the esterification of succinic acid with ethanol.
Application
DES and 1-octanol can be blended with B5 palm oil biodiesel to improve the oxygen content and achieve a greener emission of combustion gases. It may also be used as a novel and highly efficient solvent to capture carbon dioxide(CO2) which can be potentially used as a technique to reduce carbon emission.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
208.4 °F - closed cup
Flash Point(C)
98 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Performance and emission characteristics of green diesel blends containing diethyl-succinate and 1-octanol.
Phoon LY, et al.
Journal of Cleaner Production, 161, 1192-1202 (2017)
Diethyl succinate synthesis by reactive distillation
Orjuela A, et al.
Separation and Purification Technology, 88, 151-162 (2012)
Performance evaluation of CO2 capture with diethyl succinate.
Li H, et al.
Applied Energy, 200, 119-131 (2017)
M B Ashour et al.
Toxicology and applied pharmacology, 89(3), 361-369 (1987-07-01)
Treatment with 0.5% (w/w) dietary clofibrate, a peroxisome proliferator, for 14 days induced microsomal carboxylesterase activities for five substrates including malathion, clofibrate, diethylsuccinate, diethylphthalate, and p-nitrophenylacetate in liver and kidney of male Swiss-Webster mice and Sprague-Dawley rats. The induction was
Niki M Zacharias et al.
Journal of the American Chemical Society, 134(2), 934-943 (2011-12-08)
The Krebs tricarboxylic acid cycle (TCA) is central to metabolic energy production and is known to be altered in many disease states. Real-time molecular imaging of the TCA cycle in vivo will be important in understanding the metabolic basis of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service