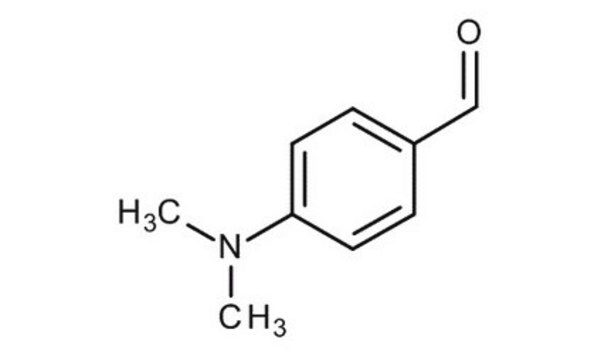
109762
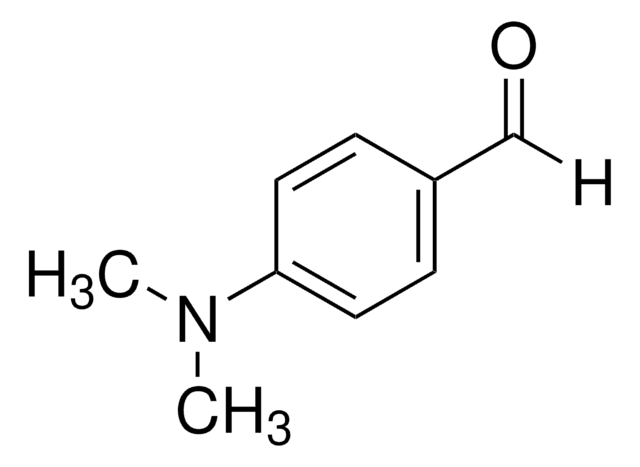
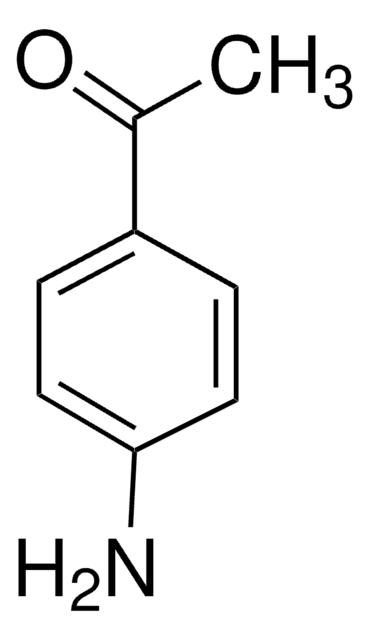
4-(Dimethylamino)benzaldehyde
98%
Synonym(s):
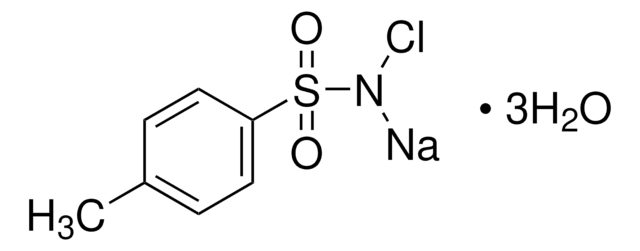
Ehrlich’s reagent
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2NC6H4CHO
CAS Number:
Molecular Weight:
149.19
Beilstein:
606802
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
72-75 °C (lit.)
solubility
H2O: slightly soluble
functional group
aldehyde
amine
SMILES string
CN(C)c1ccc(C=O)cc1
InChI
1S/C9H11NO/c1-10(2)9-5-3-8(7-11)4-6-9/h3-7H,1-2H3
InChI key
BGNGWHSBYQYVRX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-(Dimethylamino)benzaldehydeis an organic carbonyl compound containing amino and aldehyde groups. The compound is used in Ehrlich and Kovac′s reagents to test indole.4-(Dimethylamino)benzaldehyde is used to prepare aldehydes from Grignard reagents. In addition, it is used as a color test reagent for pyrroles, primary amines, and hydrazines.
Application
4-(Dimethylamino)benzaldehyde was used in the chitinase assay.
4-(Dimethylamino)benzaldehyde is used:
4-(Dimethylamino)benzaldehyde is used:
- In the synthesis of azo-azomethine dyes by condensation reaction.
- As a reagent in the synthesis of molecular adduct 4DMAB4NP (4-(dimethylamino)benzaldehyde 4-nitrophenol) by reacting with 4-nitrophenol.
- In the preparation of Schiff base.
Forms colored condensation products (Schiff bases) with pyrroles and primary amines.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Sens. 1B
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
327.2 °F - closed cup
Flash Point(C)
164 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M Legrand et al.
Proceedings of the National Academy of Sciences of the United States of America, 84(19), 6750-6754 (1987-10-01)
Four endochitinases (poly[1,4-(N-acetyl-beta-D-glucosaminide)] glycanohydrolase, EC 3.2.1.14) have been purified from leaves of Nicotiana tabacum cv. Samsun NN reacting hypersensitively to tobacco mosaic virus. Two of them are acidic proteins of molecular weights 27,500 and 28,500 and have been identified as
Synthesis, spectroscopic and TD-DFT quantum mechanical study of azo-azomethine dyes. A laser induced trans-cis-trans photoisomerization cycle
Georgiev A, et al.
Spectrochimica Acta Part A: Molecular Spectroscopy, 192, 263-274 (2018)
Synthesis, structural, spectral, third order nonlinear optical and quantum chemical investigations on hydrogen bonded novel organic molecular adduct 4-(dimethylamino) benzaldehyde 4-nitrophenol for opto-electronic applications
Karthick S, et al.
Journal of Molecular Structure, 1178, 352-365 (2019)
Jun-Min Guo et al.
Journal of agricultural and food chemistry, 58(11), 6556-6561 (2010-05-15)
A simple colorimetric method for the differentiation of indoleacetic acid (IAA) and indolebutyric acid (IBA) in plant samples is described. The color change is based upon the reaction between the auxins and p-(dimethylamino)benzaldehyde (PDAB, Ehrlich reagent) following the electrophilic substitution
Andrew P Breksa et al.
Journal of agricultural and food chemistry, 55(13), 5013-5017 (2007-06-05)
A method for estimating the total limonoid aglycone and glucoside concentrations in Citrus samples in terms of limonin and limonin glucoside equivalents is presented. The method consists of extraction followed by colorimetric quantification. The colorimetric quantification was based on the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service