517690
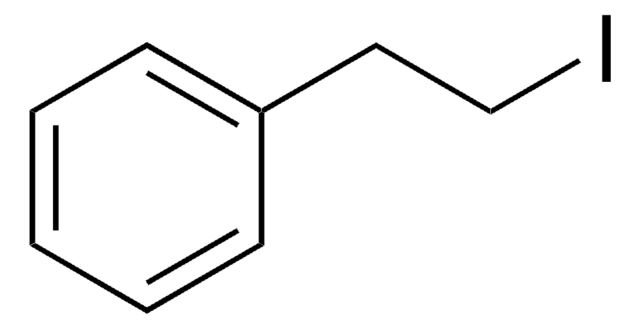
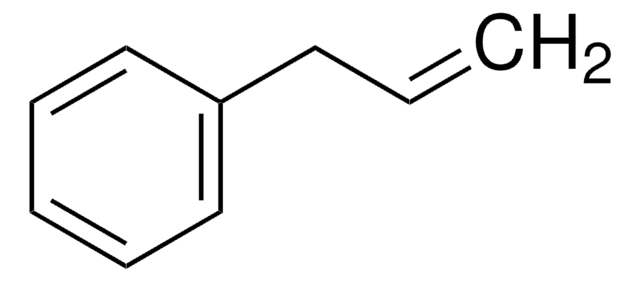
1-Iodo-3-phenylpropane
97%
Synonym(s):
3-Iodopropylbenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5(CH2)3I
CAS Number:
Molecular Weight:
246.09
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.5820 (lit.)
density
1.530 g/mL at 25 °C (lit.)
SMILES string
ICCCc1ccccc1
InChI
1S/C9H11I/c10-8-4-7-9-5-2-1-3-6-9/h1-3,5-6H,4,7-8H2
InChI key
RGCKJSPKMTWLLX-UHFFFAOYSA-N
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Thorsten Wilhelm et al.
Organic letters, 7(18), 4053-4056 (2005-08-27)
A new three-component, palladium-catalyzed domino reaction which gives access to meta-substituted arenes using aryl iodides and primary alkyl halides is reported. Various functional groups are tolerated on both the aryl iodide and alkyl halide. In addition, isotopic labeling studies provide
Y Zhang et al.
Journal of medicinal chemistry, 43(25), 4840-4849 (2000-12-22)
A series of analogues related to 1-[2-(diphenylmethoxy)ethyl]-4-(3-phenylpropyl)piperazine (2) and 1-¿2-[bis(4-fluorophenyl)methoxy]ethyl¿-4-(3-phenylpropyl)piperazine (3) (GBR 12935 and GBR 12909, respectively), in which the piperazine moiety was replaced by bridged piperazines for structural rigidity, has been designed, synthesized, and evaluated for their ability to
Synthesis of Aliphatic [carbonyl-11C] Esters Using [11C] Carbon Monoxide.
Itsenko O, et al.
European Journal of Organic Chemistry, 17, 3830-3834 (2005)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service