All Photos(1)
About This Item
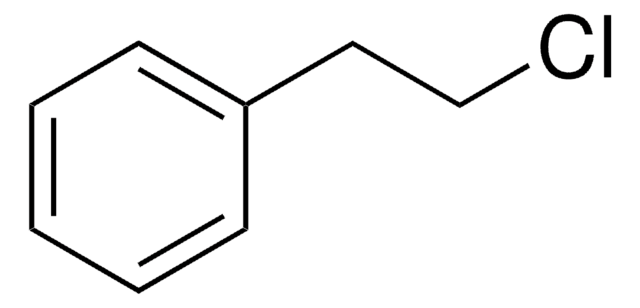
Linear Formula:
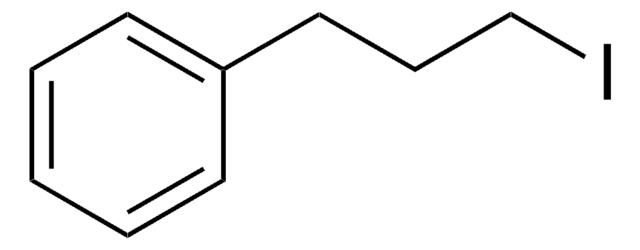
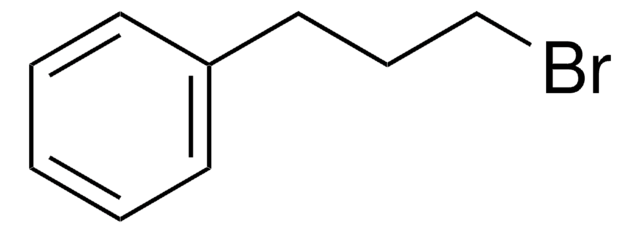
C6H5CH2CH2I
CAS Number:
Molecular Weight:
232.06
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.601 (lit.)
bp
122-123 °C/13 mmHg (lit.)
density
1.603 g/mL at 25 °C (lit.)
functional group
iodo
phenyl
SMILES string
ICCc1ccccc1
InChI
1S/C8H9I/c9-7-6-8-4-2-1-3-5-8/h1-5H,6-7H2
InChI key
KVTHPKXDLVYNCH-UHFFFAOYSA-N
General description
(2-Iodoethyl)benzene is a halogenated hydrocarbon. It is reported to undergo triethyl borane-mediated intermolecular radical addition with 2H-azirine-3-carboxylate.
Application
(2-Iodoethyl)benzene is suitable for use as starting reagent in the preparation of organic-inorganic hybrid compounds such as, [C6H5CH2NH3]2PbI4, [C6H5CH2CH2SC(NH2 )2]3PbI5 and [C10H7CH2NH3]PbI3. It may be used in the preparation of dioxane-based antiviral agents.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Intermolecular alkyl radical addition to methyl 2-(2, 6-dichlorophenyl)-2H-azirine-3-carboxylate.
Lemos A, et al.
Synlett, 10, 1403-1406 (2003)
Preparation and Characterization of [C6H5CH2NH3] 2PbI4,[C6H5CH2CH2SC (NH2) 2] 3PbI5 and [C10H7CH2NH3] PbI3 Organic-Inorganic Hybrid Compounds.
Papavassiliou GC, et al.
Zeitschrift fur Naturforschung, 54b, 1405-1405 (1999)
Ha Young Kim et al.
Bioorganic & medicinal chemistry letters, 15(13), 3207-3211 (2005-06-02)
Dioxane-based antiviral agents targeted to the hydrophobic binding pocket of Sindbis virus capsid protein were designed by computer graphics molecular modeling and synthesized. Virus production using SIN-IRES-Luc and capsid assembly were monitored to evaluate antiviral activity. A compound with a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service