63176
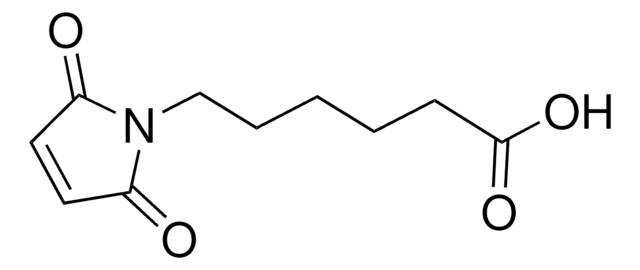
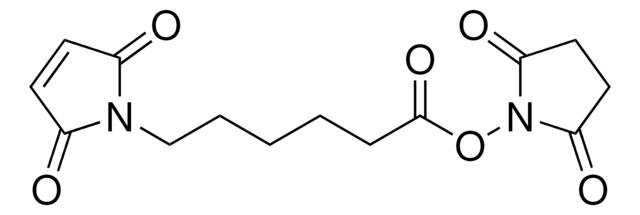
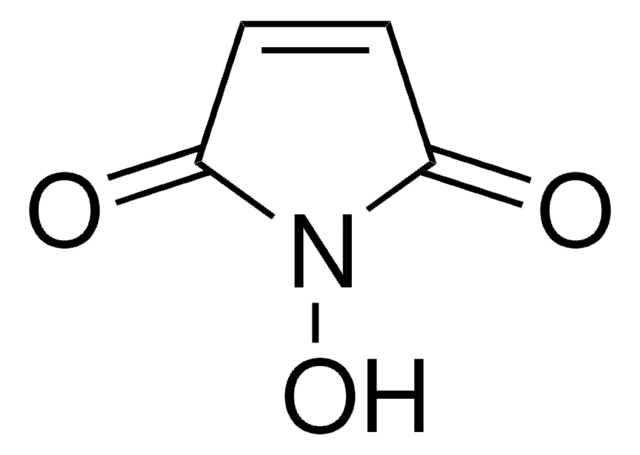
6-Maleimidohexanoic acid
≥98.0% (HPLC)
Synonym(s):
6-Maleimidocaproic acid, N-Maleoyl-6-aminocaproic acid, N-(5-Carboxypentyl)maleimide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H13NO4
CAS Number:
Molecular Weight:
211.21
Beilstein:
1532405
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Quality Level
Assay
≥98.0% (HPLC)
mp
86-91 °C
storage temp.
room temp
SMILES string
OC(=O)CCCCCN1C(=O)C=CC1=O
InChI
1S/C10H13NO4/c12-8-5-6-9(13)11(8)7-3-1-2-4-10(14)15/h5-6H,1-4,7H2,(H,14,15)
InChI key
WOJKKJKETHYEAC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
6-Maleimidohexanoic acid may be used as a spacer in the construction of drug and other types of bioconjugates. 6-Maleimidohexanoic acid is used with N-hydroxysuccinimide ester as a bifunctional cross-linking reagent.
Probe for thiol groups (SH-groups) in membrane proteins.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mitsuko Maeda et al.
Bioorganic & medicinal chemistry letters, 15(3), 621-624 (2005-01-25)
The adenovirus vector is a promising carrier for the efficient transfer of genes into cells via the coxackie-adenovirus receptor (CAR) and integrins (alphavbeta3 and alphavbeta5). The clinical use of the adenovirus vector remains problematic however. Successful administration of this vector
Shinya Kida et al.
Chemical & pharmaceutical bulletin, 55(4), 685-687 (2007-04-06)
6-maleimidohexanoic acid N-hydroxysuccinimide ester has been used widely for preparation of enzyme immunoconjugates as a unique heterobifunctional cross-linking reagent. Its heterobifunctional reactivity is good, but its ester portion hydrolyzes easily in the presence of water. Several 6-maleimidohexanoic acid active esters
Ralph Rahme et al.
Journal of neurosurgery, 121(6), 1354-1358 (2014-09-27)
The role of endovascular therapy in patients with acute ischemic stroke and a solitary M2 occlusion remains unclear. Through a pooled analysis of 3 interventional stroke trials, the authors sought to analyze the impact of successful early reperfusion of M2
Mohammed Abdelsaid et al.
Life sciences, 118(2), 268-273 (2014-01-23)
We have shown that diabetes causes cerebrovascular remodeling in part by the activation of the endothelin (ET-1) system in a glucose-dependent manner. We also reported increased yet dysfunctional cerebral angiogenesis in diabetes. Here, we tested the hypothesis that dual ET-1
S S Ghosh et al.
Bioconjugate chemistry, 1(1), 71-76 (1990-01-01)
Two general methods which exploit the reactivity of sulfhydryl groups toward maleimides are described for the synthesis of oligonucleotide-enzyme conjugates for use as nonradioisotopic hybridization probes. In the first approach, 6-maleimidohexanoic acid succinimido ester was used to couple 5'-thiolated oligonucleotide
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service