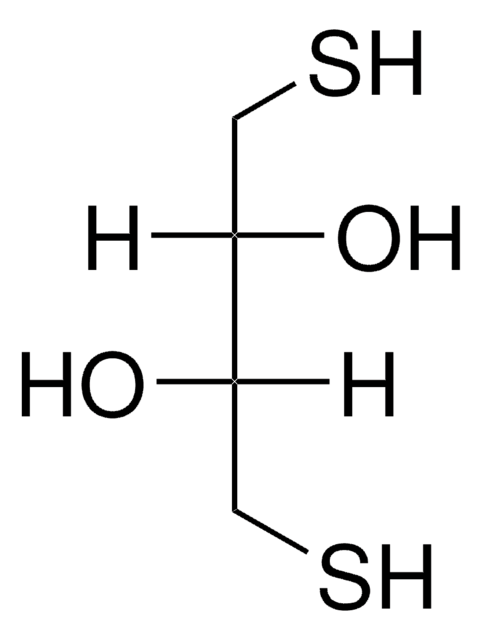
C4706
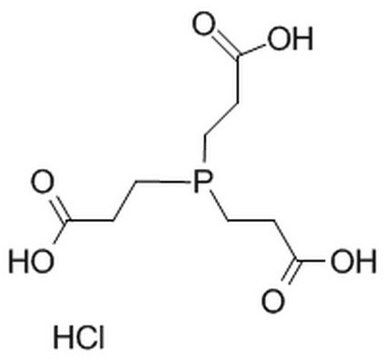
Tris(2-carboxyethyl)phosphine hydrochloride
powder
Synonym(s):
TCEP
About This Item
Recommended Products
description
Protect from moisture
Quality Level
form
powder
reaction suitability
reagent type: reductant
color
white
solubility
H2O: 50 mg/mL
storage temp.
2-8°C
SMILES string
Cl[H].OC(=O)CCP(CCC(O)=O)CCC(O)=O
InChI
1S/C9H15O6P.ClH/c10-7(11)1-4-16(5-2-8(12)13)6-3-9(14)15;/h1-6H2,(H,10,11)(H,12,13)(H,14,15);1H
InChI key
PBVAJRFEEOIAGW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- As a reducing agent for the reduction of sulfoxides, sulfonyl chlorides, N-oxides, and azides. It can also be used in azide-alkyne cycloaddition reaction in the presence of a copper catalyst.
- To reduce disulfide bonds in various proteins.
- As a reagent for the selective reduction of disulfides in water.
- To remove ruthenium-derived metathesis catalysts via aqueous washing of a crude reaction mixture when it is basified.
- As a reducing agent for the reduction of various alkyl disulfides such as trans-4,5-dihydroxy-1,2-dithiane.
Caution
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Overcome challenges in synthesis and disulfide bond formation with protocols for Fmoc solid-phase peptide synthesis of peptides with cysteine and methionine.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service