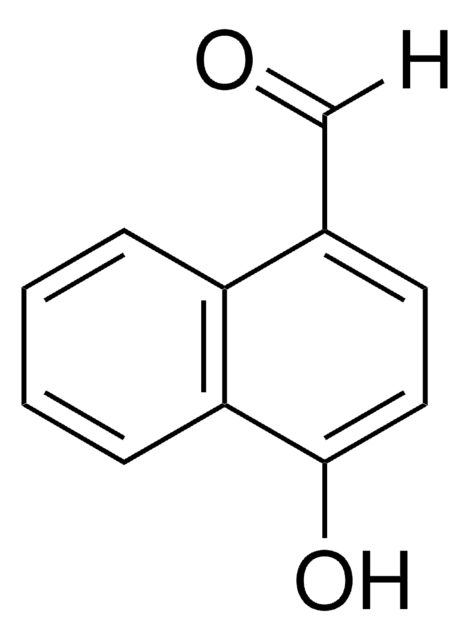
All Photos(4)
About This Item
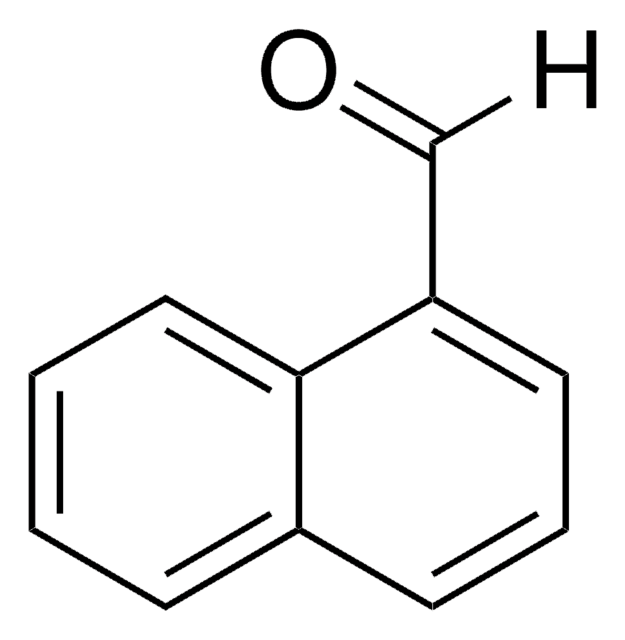
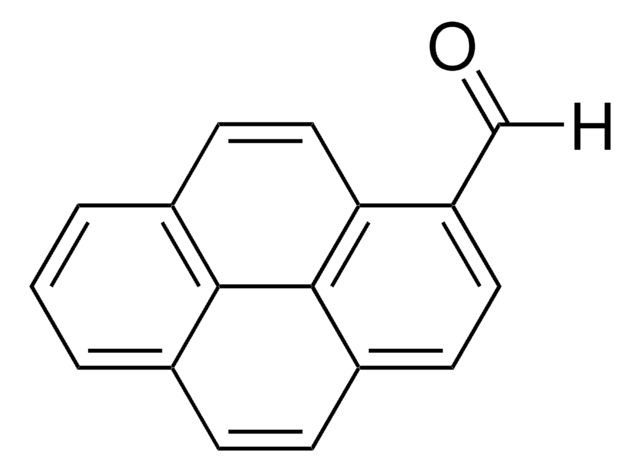
Linear Formula:
C10H7CHO
CAS Number:
Molecular Weight:
156.18
Beilstein:
507750
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
crystals
mp
58-61 °C (lit.)
storage temp.
−20°C
SMILES string
[H]C(=O)c1ccc2ccccc2c1
InChI
1S/C11H8O/c12-8-9-5-6-10-3-1-2-4-11(10)7-9/h1-8H
InChI key
PJKVFARRVXDXAD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2-Naphthaldehyde can be used as a reactant:
- In proline catalyzed aldol reaction.
- In asymmetric three-component Mannich reaction.
- For the synthesis of Hantzsch 1,4-dihydropyridines.13}
- Asymmetric benzoin condensation reaction.
- For the synthesis of pyrazolo[1,2−b]phthalazinediones.
- For the synthesis C60 by flash vacuum pyrolysis.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The direct catalytic asymmetric three-component Mannich reaction.
List B
Journal of the American Chemical Society, 122(38), 9336-9337 (2000)
Zhipeng Zhang et al.
Nature communications, 7, 12478-12478 (2016-08-18)
Due to the high versatility of chiral cyanohydrins, the catalytic asymmetric cyanation reaction of carbonyl compounds has attracted widespread interest. However, efficient protocols that function at a preparative scale with low catalyst loading are still rare. Here, asymmetric counteranion-directed Lewis
Amelioration of H4 [W12SiO40] by nanomagnetic heterogenization: For the synthesis of 1H-pyrazolo [1, 2-b] phthalazinedione derivatives.
Arora P and Rajput JK
Applied Organometallic Chemistry, 32(2), e4001-e4001 (2018)
An efficient nucleophilic carbene catalyst for the asymmetric benzoin condensation.
Enders D and Kallfass U
Angewandte Chemie (International Edition in English), 41(10), 1743-1745 (2002)
A novel TMSI-mediated synthesis of Hantzsch 1, 4-dihydropyridines at ambient temperature.
Sabitha G, et al.
Tetrahedron Letters, 44(21), 4129-4131 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service