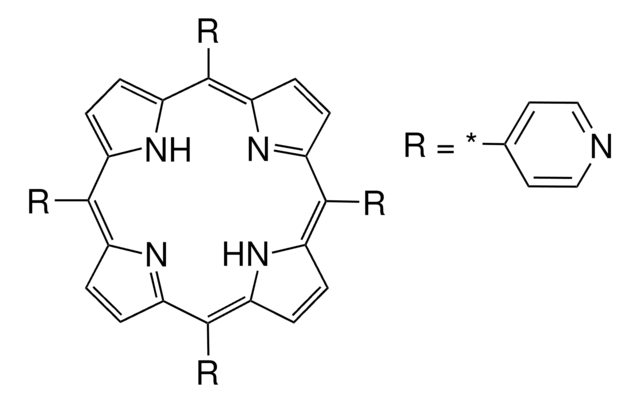
P62402
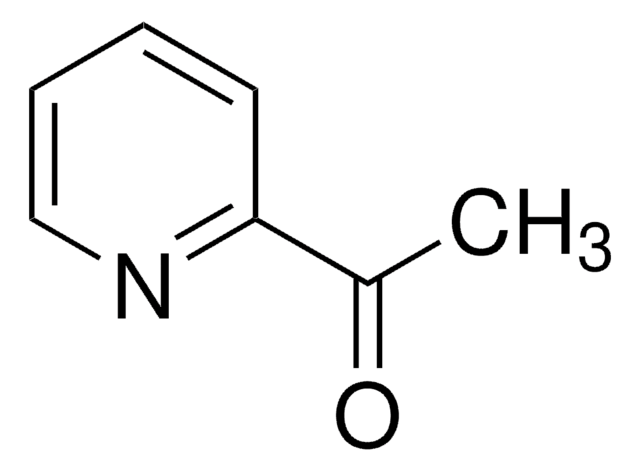
4-Pyridinecarboxaldehyde
97%
Synonym(s):
Isonicotinaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C6H5NO
CAS Number:
Molecular Weight:
107.11
Beilstein:
105342
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.544 (lit.)
bp
71-73 °C/10 mmHg (lit.)
density
1.137 g/mL at 20 °C (lit.)
storage temp.
2-8°C
SMILES string
[H]C(=O)c1ccncc1
InChI
1S/C6H5NO/c8-5-6-1-3-7-4-2-6/h1-5H
InChI key
BGUWFUQJCDRPTL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Pyridinecarboxaldehyde is a heterocyclic building block used to prepare Schiff bases via a Korich-type reaction.
Application
4-Pyridinecarboxaldehyde can be used for the synthesis of:
- ʅ,β-Unsaturated amides by coupling with N,N-disubstituted formamides.
- meso-Substituted A3-corroles.
- N-(4-pyridylmethyl)-L-valine as a ligand to construct zinc metal–organic frameworks (Zn-MOFs).
- 4′-Pyridyl terpyridines, with potential application as anticancer and antimicrobial agents.
- 4-pyridinecarboxaldehyde thiosemicarbazone, as a corrosion inhibitor for mild steel.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1B - Skin Sens. 1
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
172.0 °F
Flash Point(C)
77.8 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A combined computational/experimental study on HSA binding of two water-soluble Schiff base ligands derived from pyridine derivative and ethylendiamine.
Hajar Molaee et al.
Journal of biomolecular structure & dynamics, 37(3), 641-648 (2018-02-03)
Cross coupling of acyl and aminyl radicals: Direct synthesis of amides catalyzed by Bu4NI with TBHP as an oxidant.
Liu Z, et al.
Angewandte Chemie (International Edition in English), 51(13), 3231-3235 (2012)
Sakineh Omidi et al.
Carbohydrate polymers, 231, 115745-115745 (2020-01-01)
Hydrogels are promising carriers for the controlled drug delivery in response to the external stimuli such as pH and temperature. Here, a new hydrogel is designed and synthesized from the cross-linking of graphene, chitosan, and cellulose nanowhisker via Schiff base
Sakineh Omidi et al.
Carbohydrate polymers, 208, 477-485 (2019-01-20)
Chitosan is an antibacterial biopolymer and conjugation of it with other antimicrobial agents can be a valuable method to improve the potential application of the resultant materials in the various industries such as cosmetics, food and packaging materials. In this
Helical water chain mediated proton conductivity in homochiral metal-organic frameworks with unprecedented zeolitic unh-topology.
Sahoo SC, et al.
Journal of the American Chemical Society, 133(44), 17950-17958 (2011)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service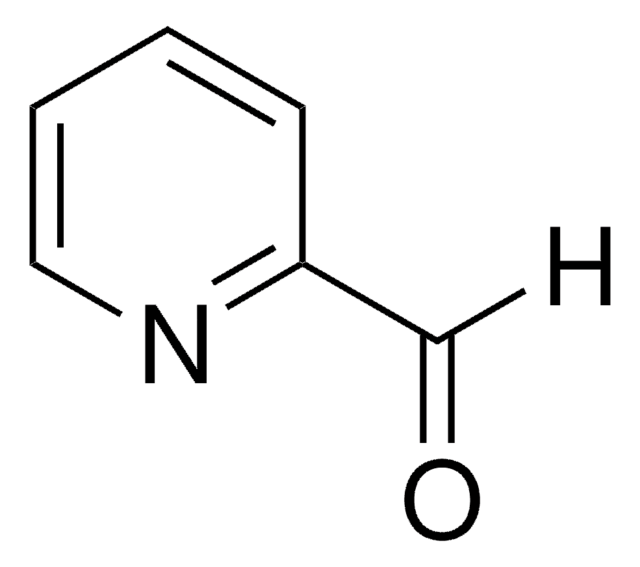

![Benzo[b]thiophene-2-carboxaldehyde 97%](/deepweb/assets/sigmaaldrich/product/structures/321/060/32405a4e-5720-4c6d-91cf-115c747270c4/640/32405a4e-5720-4c6d-91cf-115c747270c4.png)