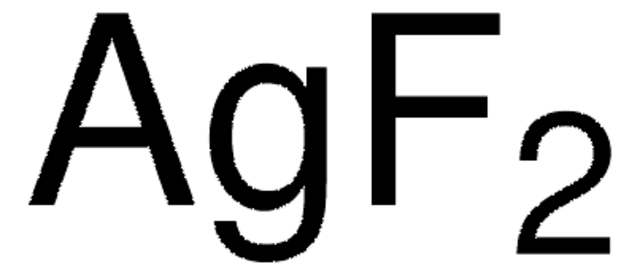217905
Copper(II) fluoride
98%
Synonym(s):
Cupric fluoride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
CuF2
CAS Number:
Molecular Weight:
101.54
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.23
grade:
for analytical purposes
form:
powder
Recommended Products
grade
for analytical purposes
Quality Level
Assay
98%
form
powder
loss
<0.5% loss on drying, 105 °C, 1h
mp
950 °C (dec.) (lit.)
density
4.23 g/mL at 25 °C (lit.)
application(s)
battery manufacturing
SMILES string
F[Cu]F
InChI
1S/Cu.2FH/h;2*1H/q+2;;/p-2
InChI key
GWFAVIIMQDUCRA-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Copper fluoride is a monoclinic weak ferromagnet. The structural properties were investigated by neutron diffraction.
Application
Copper fluoride can act as an intermediate in the green synthesis of fluoro aromatics.
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Magnetic and crystal structure of copper(II) fluoride
Fischer P, et al.
Journal of Physics and Chemistry of Solids, 35(12), 1683-1689 (1974)
T Akimoto et al.
Shika zairyo, kikai = Journal of the Japanese Society for Dental Materials and Devices, 8(3), 425-430 (1989-05-01)
As a model experiment to understand the mechanism of adhesion of the MMA/TBBO resin to dentin, polymerization of MMA/TBBO was investigated in the presence of a collagen sheet treated with the aqueous citric acid (CA) solutions of copper fluoride (CF)
J G de Oliveira Cordeiro
The Bulletin of Tokyo Medical and Dental University, 42(3), 105-116 (1995-12-01)
The aim of this investigation was to evaluate the effect of topical applications of various fluoride compounds on the development of root surface caries in hamsters. Male golden hamsters (n = 115) were divided into 7 groups and were given
"A greener" synthetic route for fluoroaromatics via copper (II) fluoride.
Subramanian MA and Manzer LE
Science, 297(5587), 1665-1665 (2002)
M Maltz et al.
Scandinavian journal of dental research, 96(5), 390-392 (1988-10-01)
Topical applications of 10 mM CuF2, 10 mM CuSO4, or 20 mM NaF solutions were performed twice a day in hamsters infected with S. mutans and maintained on a high sucrose diet. Animals receiving the copper compounds exhibited lower plaque
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service