SMB00702
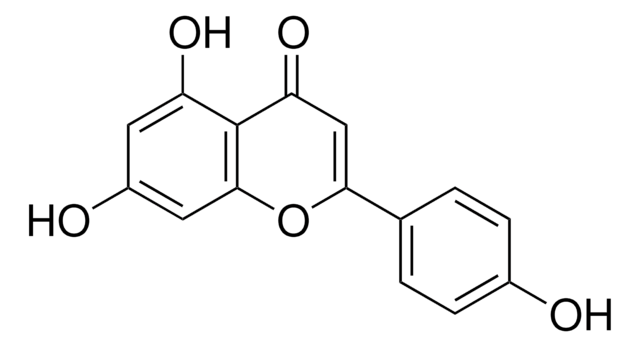
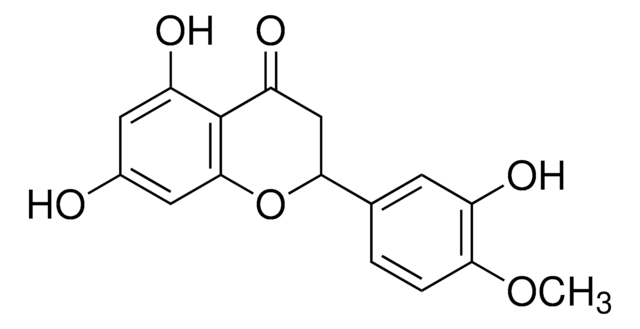
Apigenin
≥97% (TLC), from citrus
Sinónimos:
4′,5,7-Trihydroxyflavone, 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4-benzopyrone
About This Item
Productos recomendados
Quality Level
assay
≥97% (TLC)
form
powder
mp
>300 °C (lit.)
solubility
DMSO: 27 mg/mL
1 M KOH: 50 mg/mL
application(s)
metabolomics
vitamins, nutraceuticals, and natural products
storage temp.
−20°C
SMILES string
Oc1ccc(cc1)C2=CC(=O)c3c(O)cc(O)cc3O2
InChI
1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H
InChI key
KZNIFHPLKGYRTM-UHFFFAOYSA-N
Gene Information
human ... ADORA3(140) , CDC2(983) , CDK5(1020) , CDK6(1021) , CYP1A2(1544) , ESR1(2099) , ESR2(2100) , GSK3A(2931)
mouse ... Hexa(15211)
rat ... Adora1(29290) , Adora2a(25369) , IL4(287287) , Tnf(24835)
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
- to test its anti-cancer effects as a reference agent to treat various digestive track and liver cancer cells in in vitro studies
- as stimuli and inhibitor to test its anti-inflammatory effects on in vitro inflammatory responses due to dietary and metabolic changes of rural- and urban-living Tanzanians
- as a reference standard to identify the phenolic compounds in olive mill wastewater and pomace
Biochem/physiol Actions
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico