G6649
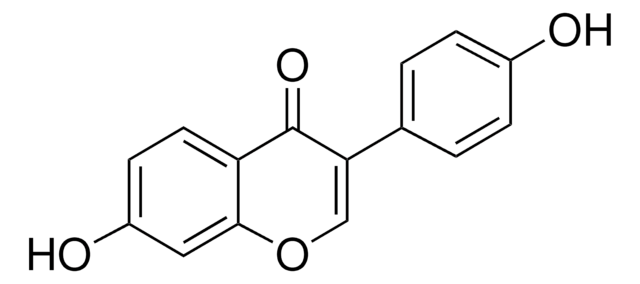
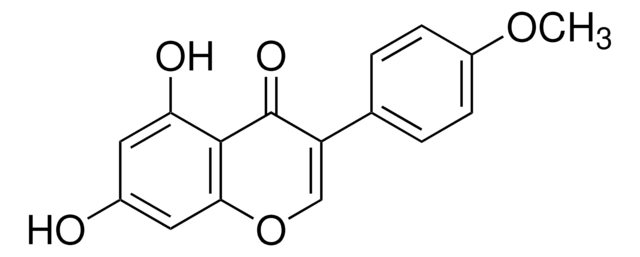
Genistein
≥98% (HPLC), powder, tyrosine protein kinase inhibitor
Sinónimos:
4′,5,7-Trihydroxyisoflavone, 5,7-Dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one
About This Item
Productos recomendados
Nombre del producto
Genistein, synthetic, ≥98% (HPLC), powder
biological source
synthetic
Quality Level
assay
≥98% (HPLC)
form
powder
color
off-white to yellow
mp
297.0-298.0 °C
solubility
DMSO: soluble
ethanol: soluble
storage temp.
−20°C
SMILES string
Oc1ccc(cc1)C2=COc3cc(O)cc(O)c3C2=O
InChI
1S/C15H10O5/c16-9-3-1-8(2-4-9)11-7-20-13-6-10(17)5-12(18)14(13)15(11)19/h1-7,16-18H
InChI key
TZBJGXHYKVUXJN-UHFFFAOYSA-N
Gene Information
human ... AKT1(207) , CYP19A1(1588) , EGFR(1956) , ESR1(2099) , ESR2(2100)
mouse ... Esr1(13982) , Hexa(15211)
rat ... Adora1(29290) , Adora2a(25369) , Ar(24208)
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
- as a test compound to access its estrogeniic acivity
- as an oestrogenic ligand to carry out identical reporter gene activation assays and also used to examine the binding of genistein to hERβ
- as an endocytosis inhibitor to test the possible effect of endocytosed advanced glycation end - bovine serum albumin (AGE-BSA) on lysosomes
Biochem/physiol Actions
Features and Benefits
Preparation Note
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Contenido relacionado
Discover Bioactive Small Molecules for Kinase Phosphatase Biology
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico