C4606
Anti-Calreticulin antibody produced in rabbit
IgG fraction of antiserum, buffered aqueous solution
About This Item
Productos recomendados
biological source
rabbit
conjugate
unconjugated
antibody form
IgG fraction of antiserum
antibody product type
primary antibodies
clone
polyclonal
form
buffered aqueous solution
mol wt
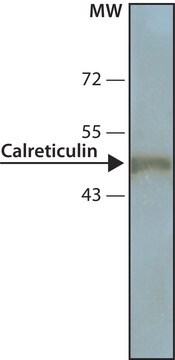
antigen 50 kDa
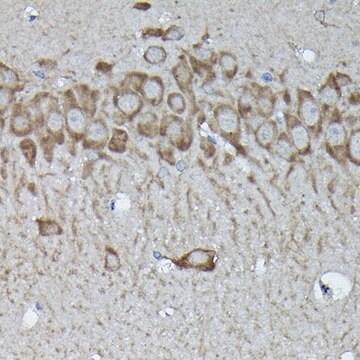
species reactivity
human, canine
technique(s)
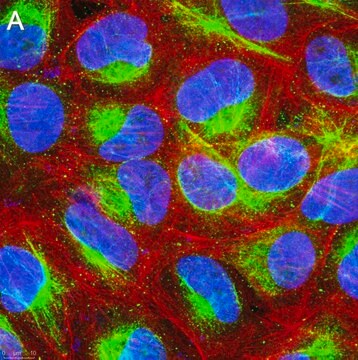
immunoprecipitation (IP): suitable using whole cell RIPA lysate of the human epitheloid carcinoma HeLa cell line
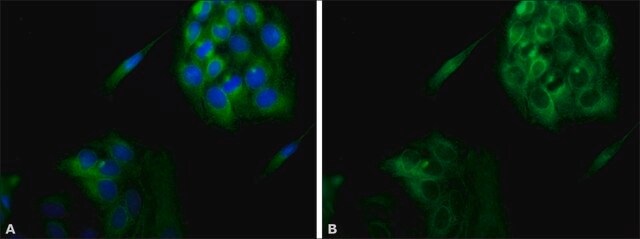
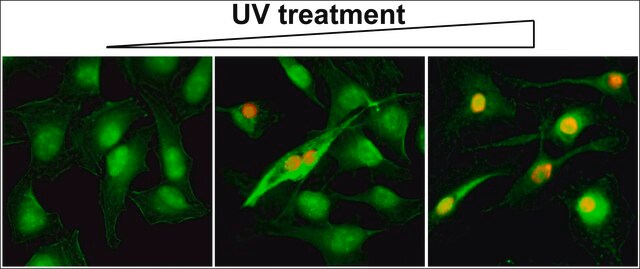
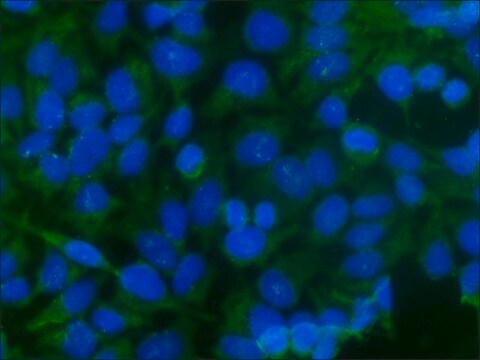
indirect immunofluorescence: 1:100 using 3% paraformaldehyde-fixed, 0.5% Triton X-100 treated, Madin Darby canine kidney MDCK cell line
microarray: suitable
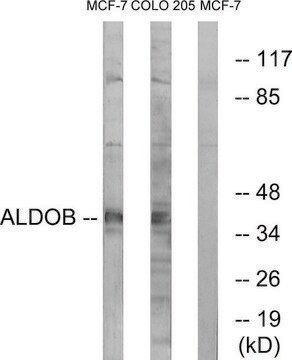
western blot: 1:2,000 using whole cell RIPA lysate of the human epitheloid carcinoma HeLa cell line
UniProt accession no.
shipped in
dry ice
storage temp.
−20°C
target post-translational modification
unmodified
Gene Information
human ... CALR(811)
General description
Specificity
Immunogen
Application
- immunoblotting
- immunofluorescence
- immunocytochemistry{91
- immunoprecipitation
Biochem/physiol Actions
Physical form
Storage and Stability
Disclaimer
¿No encuentra el producto adecuado?
Pruebe nuestro Herramienta de selección de productos.
Optional
related product
Storage Class
10 - Combustible liquids
wgk_germany
nwg
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico