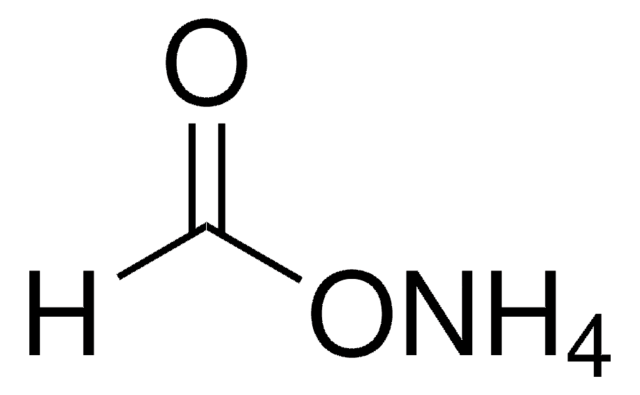
584222
Tetrahydrouridine
Potent competitive inhibitor of cytidine deaminase. Also available as a 100 mM solution in H2O.
Sinónimos:
Tetrahydrouridine
About This Item
Productos recomendados
Quality Level
assay
≥90% (TLC)
form
solid
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
desiccated (hygroscopic)
protect from light
color
white to off-white
solubility
water: 200 mg/mL
shipped in
wet ice
storage temp.
−20°C
InChI
1S/C9H16N2O6/c12-3-4-6(14)7(15)8(17-4)11-2-1-5(13)10-9(11)16/h4-8,12-15H,1-3H2,(H,10,16)/t4-,5?,6-,7-,8-/m1/s1
InChI key
UCKYOOZPSJFJIZ-XVKVHKPRSA-N
Categorías relacionadas
General description
Application
- Oncotherapy resistance explained by Darwinian and Lamarckian models.: This research explores the mechanisms of oncotherapy resistance, combining Darwinian and Lamarckian models to provide a comprehensive understanding. The study highlights the role of Tetrahydrouridine in modulating therapy resistance pathways in cancer treatment (Saunthararajah et al., 2024).
- Neuroendocrine lineage commitment of small cell lung cancers can be leveraged into p53-independent non-cytotoxic therapy.: This study investigates the potential of using Tetrahydrouridine in non-cytotoxic therapies for small cell lung cancers, focusing on neuroendocrine lineage commitment and its implications for treatment efficacy (Biswas et al., 2023).
- In Vitro Interaction of Tetrahydrouridine with Key Human Nucleoside Transporters.: This study explores how Tetrahydrouridine interacts with human nucleoside transporters, shedding light on its mechanisms of action and potential as a therapeutic agent in biochemistry and pharmacology (Säll et al., 2023).
- Mycoplasma infection of cancer cells enhances anti-tumor effect of oxidized methylcytidines.: This research investigates how mycoplasma infection enhances the anti-tumor effects of oxidized methylcytidines, with Tetrahydrouridine playing a crucial role in the observed therapeutic outcomes (Pang et al., 2023).
Packaging
Warning
Reconstitution
Other Notes
Bouffard, D.Y., et al. 1993. Biochem Pharmacol.45, 1857.
Laliberte, J., et al. 1992. Cancer Chemotherap. Pharmacol.30, 7.
Riva, C., et al. 1992. Chemotherapy38, 358.
Yusa, K., et al. 1992. J. Biol. Chem. 267, 16848.
Hanze, A.R. 1967. J. Am. Chem. Soc.89, 6720.
Legal Information
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico