W326305
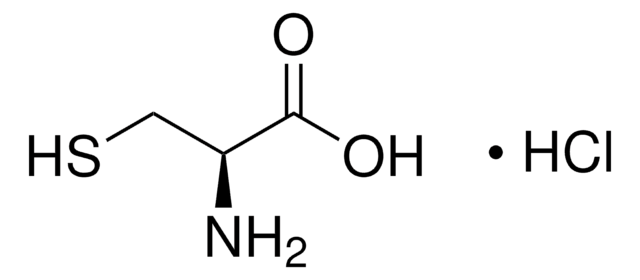
L-cisteína
≥97%, FG
Sinónimos:
Ácido (R)-2-amino-3-mercaptopropiónico
About This Item
Productos recomendados
grade
FG
Halal
reg. compliance
EU Regulation 1334/2008 & 872/2012
FDA 21 CFR 172.320
FDA 21 CFR 184.1271
assay
≥97%
optical activity
[α]26/D +8.0 to +9.5°, c = 2 in 5 M HCl
mp
240 °C (dec.) (lit.)
solubility
H2O: 25 mg/mL
application(s)
flavors and fragrances
documentation
see Safety & Documentation for available documents
organoleptic
sulfurous
SMILES string
N[C@@H](CS)C(O)=O
InChI
1S/C3H7NO2S/c4-2(1-7)3(5)6/h2,7H,1,4H2,(H,5,6)/t2-/m0/s1
InChI key
XUJNEKJLAYXESH-REOHCLBHSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Biochem/physiol Actions
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 1
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Protocolos
Separation of L-Alanine; Glycine; L-Valine; L-Leucine; L-Isoleucine; L-Proline; L-Methionine; L-Serine; L-Threonine; L-Phenylalanine; L-Aspartic acid; L-4-Hydroxyproline; L-Cysteine; L-Glutamic acid; L-Asparagine; L-Lysine; L-Glutamine; L-Histidine; L-Tyrosine; L-Tryptophan; L-Cystine
Chromatograms
application for HPLCNuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico