W304522
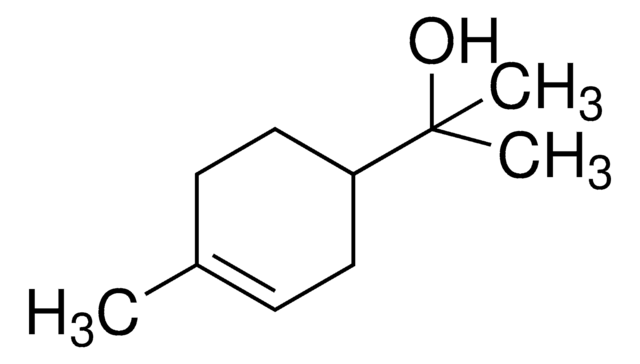
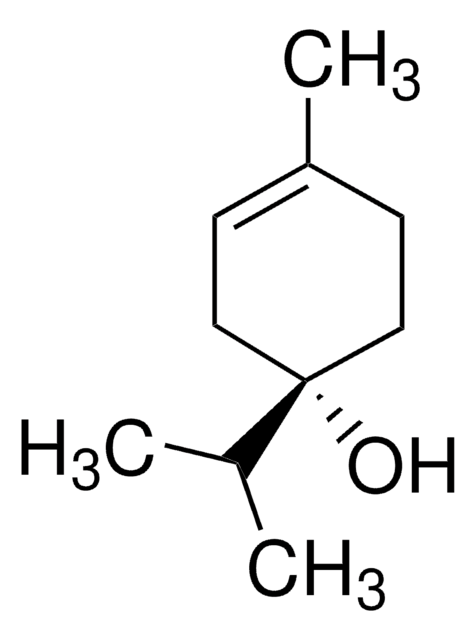
(−)−α-Terpineol
natural, ≥96%, FCC, FG
Sinónimos:
(S)-2-(4-Methyl-3-cyclohexenyl)-2-propanol, (S)-p-Menth-1-en-8-ol
About This Item
Fragrance grade
Halal
Kosher
natural
Productos recomendados
grade
FG
Fragrance grade
Halal
Kosher
natural
Quality Level
agency
follows IFRA guidelines
reg. compliance
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FCC
FDA 21 CFR 172.515
FDA 21 CFR 178.1010
assay
≥96%
greener alternative product characteristics
Less Hazardous Chemical Syntheses
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
refractive index
n20/D 1482 (lit.)
bp
217-218 °C (lit.)
mp
31-35 °C (lit.)
density
0.93 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
documentation
see Safety & Documentation for available documents
food allergen
no known allergens
fragrance allergen
α-terpineol
greener alternative category
organoleptic
lilac; citrus; woody; floral; pine
SMILES string
CC1=CC[C@@H](C(O)(C)C)CC1
InChI
1S/C10H18O/c1-8-4-6-9(7-5-8)10(2,3)11/h4,9,11H,5-7H2,1-3H3/t9-/m1/s1
InChI key
WUOACPNHFRMFPN-SECBINFHSA-N
Categorías relacionadas
General description
Application
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
204.8 °F - closed cup
flash_point_c
96 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
-Cymene; Linalool; Menthol; α-Terpineol; Menthyl acetate
Protocolos
-(+)-Limonene, purum, ≥98.0% (sum of enantiomers, GC); Geranyl tiglate; α-Terpineol, natural, ≥96%, FCC, FG; Geranyl formate; α-Pinene
-Pinocarveol; Menthol; (+)-Terpinen-4-ol; α-Terpineol; (±)-α-Terpinyl acetate, predominantly α-isomer; Germacrene D
-Cymene; (−)-Menthone; α-Terpineol, natural, ≥96%, FCC, FG; Terpinolene; β-Bourbonene; 1-Octen-3-ol; β-Caryophyllene; Linalool; α-Terpinene; (−)-Menthol
-α-Bergamotene; β-Bisabolene; α-Terpineol; Neryl acetate; Geranyl acetate; Neral; Geranial
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico