T84654
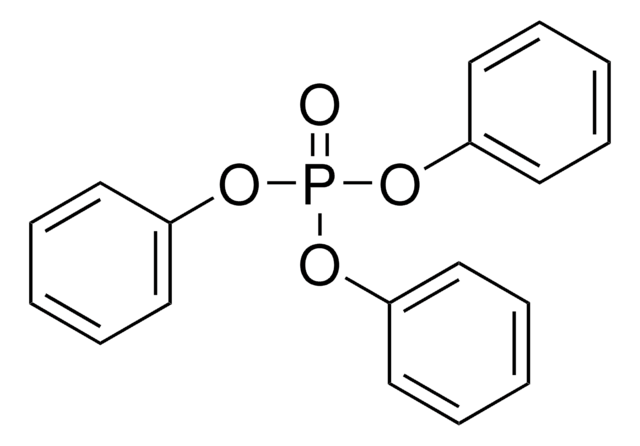
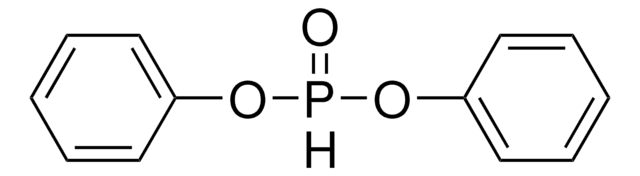
Triphenyl phosphite
97%
Sinónimos:
(PhO)3P, P(OPh)3, Triphenoxyphosphine
About This Item
Productos recomendados
vapor density
10.7 (vs air)
Quality Level
vapor pressure
5 mmHg ( 205 °C)
assay
97%
reaction suitability
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: ligand
reaction type: Alkylations
reagent type: ligand
reaction type: Cycloadditions
reagent type: ligand
reaction type: Heck Reaction
reagent type: ligand
reaction type: Olefinations
reagent type: ligand
reaction type: Stille Coupling
reagent type: ligand
reaction type: Wittig Reaction
refractive index
n20/D 1.59 (lit.)
bp
360 °C (lit.)
mp
22-24 °C (lit.)
density
1.184 g/mL at 25 °C (lit.)
SMILES string
O(P(Oc1ccccc1)Oc2ccccc2)c3ccccc3
InChI
1S/C18H15O3P/c1-4-10-16(11-5-1)19-22(20-17-12-6-2-7-13-17)21-18-14-8-3-9-15-18/h1-15H
InChI key
HVLLSGMXQDNUAL-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
- As a source of phosphorus and as a ligand for the synthesis of transition metal phosphide nanoparticles via heating-up process.
- To convert alcohols to alkyl halides.
- As a peptide coupling agent.
- As a low-temperature source of singlet oxygen after forming an adduct with ozone.
- To synthesize bromotriphenoxyphosphonium bromide, a brominating agent, by reacting with bromine.
signalword
Warning
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT RE 2
target_organs
Nervous system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 2
flash_point_f
410.0 °F
flash_point_c
210 °C
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico