M14900
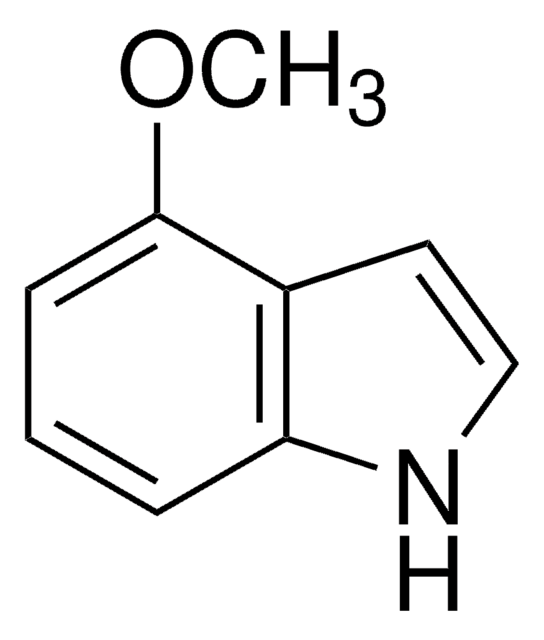
5-Methoxyindole
99%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C9H9NO
Número de CAS:
Peso molecular:
147.17
Beilstein/REAXYS Number:
116722
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
99%
bp
176-178 °C/17 mmHg (lit.)
mp
52-55 °C (lit.)
SMILES string
COc1ccc2[nH]ccc2c1
InChI
1S/C9H9NO/c1-11-8-2-3-9-7(6-8)4-5-10-9/h2-6,10H,1H3
InChI key
DWAQDRSOVMLGRQ-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Christian Brand et al.
The Journal of chemical physics, 133(2), 024303-024303 (2010-07-17)
Rotationally resolved electronic spectra of the vibrationless origin and of eight vibronic bands of 5-methoxyindole (5MOI) have been measured and analyzed using an evolutionary strategy approach. The experimental results are compared to the results of ab initio calculations. All vibronic
Kenichiro Todoroki et al.
Analytical sciences : the international journal of the Japan Society for Analytical Chemistry, 22(2), 281-286 (2006-03-04)
In this paper we describe a highly sensitive and selective liquid chromatographic method for the determination of 5-methoxyindoles (5-methoxyindole-3-acetic acid, 5-methoxytryptamine, 5-methoxytryptophol, and melatonin) using a post-column technique involving electrolytic demethylation followed by fluorescence derivatization with benzylamine. We separated these
Pawel Sledz et al.
Journal of the American Chemical Society, 132(13), 4544-4545 (2010-03-18)
Fragment-based methods are a new and emerging approach for the discovery of protein binders that are potential new therapeutic agents. Several ways of utilizing structural information to guide the inhibitor assembly have been explored to date. One of the approaches
F Peter Guengerich et al.
Journal of medicinal chemistry, 47(12), 3236-3241 (2004-05-28)
Indigoids, a class of bis-indoles, represent a promising protein kinase inhibitor scaffold. Oxidation of indole by cytochrome P450 (P450) has been shown to generate species (indoxyl, isatin) that couple to yield indigo and indirubin. Escherichia coli-expressed human P450 2A6 mutants
Xiang-Qun Hu et al.
The Journal of biological chemistry, 283(11), 6826-6831 (2008-01-12)
Current receptor theory suggests that there is an equilibrium between the inactive (R) and active (R*) conformations of ligand-gated ion channels and G protein-coupled receptors. The actions of ligands in both receptor types could be appropriately explained by this two-state
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico