C47604
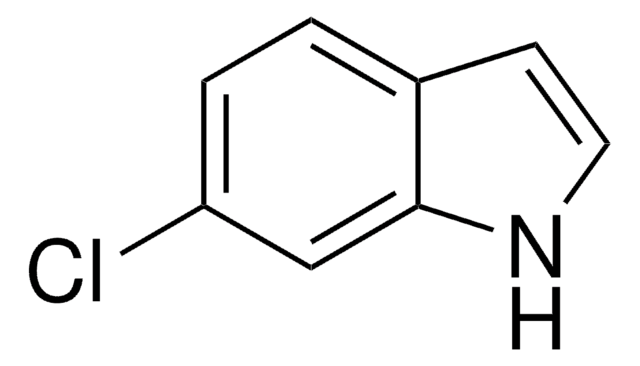
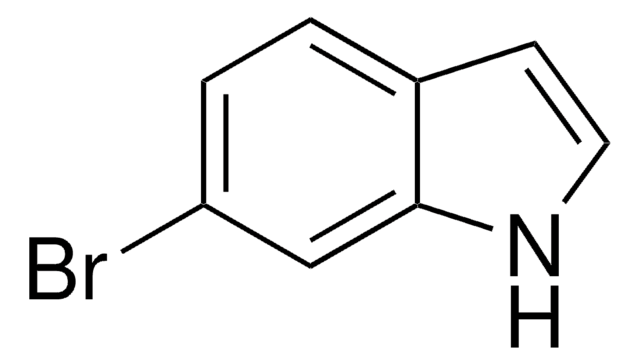
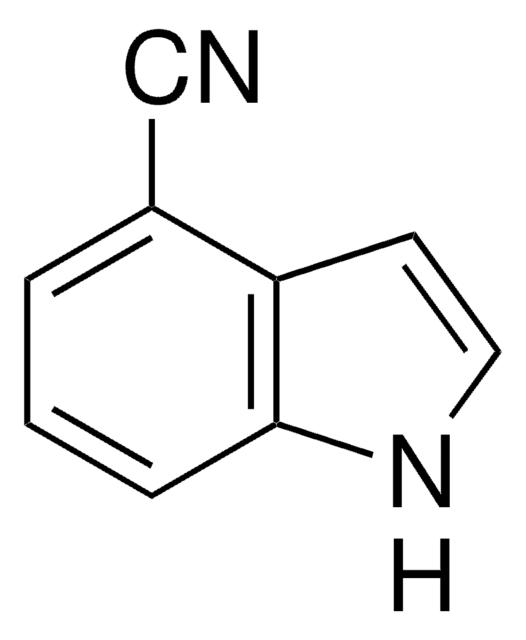
5-Chloroindole
98%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C8H6ClN
Número de CAS:
Peso molecular:
151.59
Beilstein/REAXYS Number:
2651
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
98%
form
crystals
mp
69-71 °C (lit.)
SMILES string
Clc1ccc2[nH]ccc2c1
InChI
1S/C8H6ClN/c9-7-1-2-8-6(5-7)3-4-10-8/h1-5,10H
InChI key
MYTGFBZJLDLWQG-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
5-Chloroindole can be synthesized by using 3-chlorobenzaldehyde as starting reagent.
5-Chloroindole is a 5-substituted indole. It undergoes electropolymerization to form a redox-active film consisting of a cyclic trimer and chains of linked cyclic trimer (polymer). It is a potential positive allosteric modulator (PAM) of the 5-HT3 receptor. It has been reported as strong inhibitor of the copper dissolution in acidic sodium chloride solution. It has been tested as corrosion inhibitor of mild steel in 1N deaerated sulphuric acid. Synthesis of 5-chloroindole, via nitration of indoline has been described.
Application
5-Chloroindole has been used in the synthesis of 5-chloro-3-indole-N,N- dimethylglyoxalamide and 5-chloro-N,N-dimethyltryptamine. It may be used in the synthesis of dyestuffs in the presence of biocatalysts (Escherichia coli expressing multicomponent phenol hydroxylase (mPH) isolated from Pseudomonas sp. strains KL33 and KL28).
5-Chloroindole has been used to study the biotransformation of substituted indoles to indican derivatives in the tissue cultures of Polygonum tinctorium. It may be employed as a monomer in the preparation of redox-active film made up of a cyclic trimer and chains of linked cyclic trimer (polymer).
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Indole and 5-chloroindole as inhibitors of anodic dissolution and cathodic deposition of copper in acidic chloride solutions.
Scendo M, et al.
J. Appl. Electrochem., 33(3-4), 287-293 (2003)
Fluorescence properties of electropolymerised 5-substituted indoles in solution.
Jennings P, et al.
J. Chem. Soc., Faraday Trans., 94(24), 3619-3624 (1998)
Xiaoxue Tong et al.
Microbial cell factories, 15(1), 180-180 (2016-10-23)
Engineering of single-species biofilms for enzymatic generation of fine chemicals is attractive. We have recently demonstrated the utility of an engineered Escherichia coli biofilm as a platform for synthesis of 5-halotryptophan. E. coli PHL644, expressing a recombinant tryptophan synthase, was
Synthesis of Some 5-and 6-Chloro, 5-Methyl, and 5, 6, 7-Trimethyl Derivatives of Tryptamine.
Benington F, et al.
The Journal of Organic Chemistry, 25(9), 1542-1547 (1960)
5-Amino-and 5-chloro-indole as mild steel corrosion inhibitors in 1 N sulphuric acid.
Moretti G, et al.
Electrochimica Acta, 41(13), 1971-1980 (1996)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico