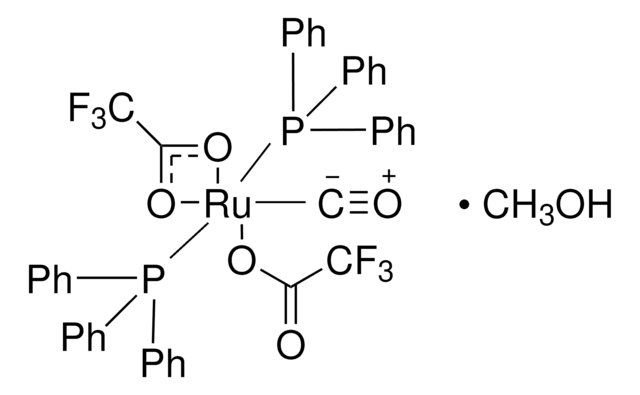
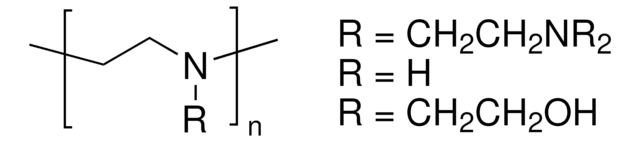901235
TBS-DHG Catalyst
≥95%
Sinónimos:
(2R,3S,4R)-2-(((tert-Butyldimethylsilyl)oxy)methyl)tetrahydro-2H-pyran-3,4-diol, 6-Tertbutyldimethylsilyl-1,2-dihydroglucal
About This Item
Productos recomendados
Quality Level
assay
≥95%
form
powder or crystals
reaction suitability
reagent type: catalyst
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
greener alternative category
, Aligned
storage temp.
−20°C
SMILES string
[H]C1([H])C([H])([H])O[C@@](C([H])([H])O[Si](C([H])([H])[H])(C([H])([H])[H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H])([H])[C@](O[H])([H])[C@]1([H])O[H]
InChI
1S/C12H26O4Si/c1-12(2,3)17(4,5)16-8-10-11(14)9(13)6-7-15-10/h9-11,13-14H,6-8H2,1-5H3/t9-,10-,11+/m1/s1
InChI key
KKFGQPZYDWCKRC-MXWKQRLJSA-N
Categorías relacionadas
General description
Application
Other Notes
- Carbohydrate-Catalyzed Enantioselective Alkene Diboration:Enhanced Reactivity of 1,2-Bonded Diboron Complexes
- Diols, α-Ketols, and Diones as 22π Components in [2+2+2] Cycloadditions of 1,6-Diynes via Ruthenium(0)-Catalyzed Transfer Hydrogenation
- Carbohydrate/DBU Cocatalyzed Alkene Diboration: Mechanistic Insight Provides Enhanced Catalytic Efficiency and Substrate Scope
related product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
Enantioselective alkene diboration is a valuable strategy for transforming unsaturated hydrocarbons into useful chiral building blocks.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico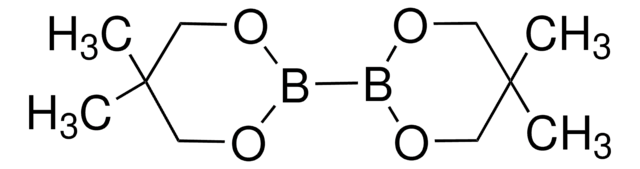
![1,8-Diazabiciclo[5.4.0]undec-7-eno 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)
![Dichloro[9,9-dimethyl-4,5-bis(diphenylphosphino)xanthene]palladium(II) 95%](/deepweb/assets/sigmaaldrich/product/structures/374/597/f7932c5b-0448-498b-8254-f8ce1b9a4612/640/f7932c5b-0448-498b-8254-f8ce1b9a4612.png)