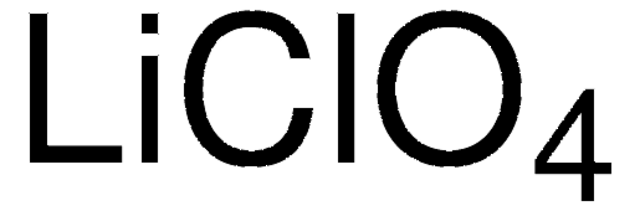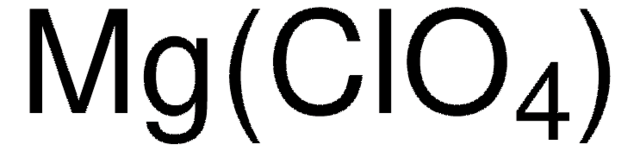431567
Lithium perchlorate
99.99% trace metals basis
Sinónimos:
Perchloric acid, lithium salt
About This Item
Productos recomendados
grade
ACS reagent
assay
99.99% trace metals basis
form
granular
reaction suitability
reagent type: oxidant
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
impurities
≤0.005% insolubles
<100 ppm total metallic impurities
pH
6.0-7.5
mp
236 °C (lit.)
solubility
H2O: 106.4 g/L at 20 °C
anion traces
chloride (Cl-): ≤0.003%
sulfate (SO42-): ≤0.001%
greener alternative category
SMILES string
[Li+].[O-]Cl(=O)(=O)=O
InChI
1S/ClHO4.Li/c2-1(3,4)5;/h(H,2,3,4,5);/q;+1/p-1
InChI key
MHCFAGZWMAWTNR-UHFFFAOYSA-M
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
- As a precursor to prepare solid polymer electrolytes for rechargeable Li-ion batteries.
- As an oxidizer to prepare polymer-based solid propellants.
- To fabricate cobalt sulfide (CoS)-based counter electrode for dye-sensitized solar cells(DSSC).
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Ox. Sol. 2 - Skin Corr. 1A - STOT SE 3
target_organs
Respiratory system
Storage Class
5.1A - Strongly oxidizing hazardous materials
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico